Glycosylation and Serological Reactivity of an Expression-enhanced SARS-CoV-2 Viral Spike Mimetic
- PMID: 34717971
- PMCID: PMC8550889
- DOI: 10.1016/j.jmb.2021.167332
Glycosylation and Serological Reactivity of an Expression-enhanced SARS-CoV-2 Viral Spike Mimetic
Abstract
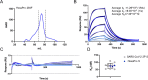
Extensive glycosylation of viral glycoproteins is a key feature of the antigenic surface of viruses and yet glycan processing can also be influenced by the manner of their recombinant production. The low yields of the soluble form of the trimeric spike (S) glycoprotein from SARS-CoV-2 has prompted advances in protein engineering that have greatly enhanced the stability and yields of the glycoprotein. The latest expression-enhanced version of the spike incorporates six proline substitutions to stabilize the prefusion conformation (termed SARS-CoV-2 S HexaPro). Although the substitutions greatly enhanced expression whilst not compromising protein structure, the influence of these substitutions on glycan processing has not been explored. Here, we show that the site-specific N-linked glycosylation of the expression-enhanced HexaPro resembles that of an earlier version containing two proline substitutions (2P), and that both capture features of native viral glycosylation. However, there are site-specific differences in glycosylation of HexaPro when compared to 2P. Despite these discrepancies, analysis of the serological reactivity of clinical samples from infected individuals confirmed that both HexaPro and 2P protein are equally able to detect IgG, IgA, and IgM responses in all sera analysed. Moreover, we extend this observation to include an analysis of glycan engineered S protein, whereby all N-linked glycans were converted to oligomannose-type and conclude that serological activity is not impacted by large scale changes in glycosylation. These observations suggest that variations in glycan processing will not impact the serological assessments currently being performed across the globe.
Keywords: SARS-CoV-2; antibody; glycoprotein; glycosylation; serology.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests J.S.M. is an inventor on the following U.S. patent applications: no. 62/412,703 (“Prefusion Coronavirus Spike Proteins and Their Use”); no. 62/972,886 (“2019-nCoV Vaccine”); no. 63/032,502 (“Engineered Coronavirus Spike (S) Protein and Methods of Use Thereof”).
Figures




References
-
- Hyakumura M., Walsh R., Thaysen-Andersen M., Kingston N.J., La M., Lu L., Lovrecz G., Packer N.H., et al. Modification of Asparagine-Linked Glycan Density for the Design of Hepatitis B Virus Virus-Like Particles with Enhanced Immunogenicity. J. Virol. 2015;89:11312–11322. doi: 10.1128/JVI.01123-15. - DOI - PMC - PubMed
-
- Pritchard L.K., Spencer D.I.R., Royle L., Vasiljevic S., Krumm S.A., Doores K.J., Crispin M. Glycan Microheterogeneity at the PGT135 Antibody Recognition Site on HIV-1 gp120 Reveals a Molecular Mechanism for Neutralization Resistance. J. Virol. 2015;89:6952–6959. doi: 10.1128/JVI.00230-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

