Development and validation of a prognostic model for leflunomide discontinuation with abnormal blood tests during long-term treatment: cohort study using data from the Clinical Practice Research Datalink Gold and Aurum
- PMID: 34718430
- PMCID: PMC9258529
- DOI: 10.1093/rheumatology/keab790
Development and validation of a prognostic model for leflunomide discontinuation with abnormal blood tests during long-term treatment: cohort study using data from the Clinical Practice Research Datalink Gold and Aurum
Abstract
Objective: To develop and validate a prognostic model for LEF discontinuation with abnormal blood test results.
Methods: Data from the Clinical Practice Research Datalink Gold and Aurum were used for model development and external validation, respectively. Participants prescribed LEF between 1 January 2007 and 31 December 2019 were followed up from 6 months after the first general practitioner prescription to the earliest of date of outcome, death, 5 year follow-up or 31 December 2019. Candidate prognostic factors were ascertained using theory and data-driven approaches. Penalized Cox regression was performed to develop the risk equation, followed by internal validation using 500 bootstraps to correct for optimism. Multiple imputation was applied to handle missing data. Model performance was assessed in terms of calibration and discrimination.
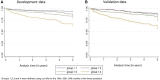
Results: Data for 1487 and 2329 participants contributing 3140 and 5246 person-years follow-up were included in the development and validation cohorts, respectively. Thirteen candidate predictors were included in the model. Epilepsy and either cytopenia or elevated liver enzymes during the first 6 months of shared-care LEF prescription were strong predictors of drug discontinuation with a hazard ratio of 4.39 (95% CI 1.74, 11.06) and 3.06 (2.15, 4.35), respectively. The unadjusted and optimism-adjusted calibration slope in development data was 1.00 (95% CI 0.75, 1.25) and 0.72 (95% CI 0.47, 0.97), respectively. The calibration slope in validation data was 0.91 (95% CI 0.74, 1.07). The model showed prognostic separation with an optimism-adjusted Royston D statistic of 0.73 (95% CI 0.44, 1.02).
Conclusion: We have developed and externally validated an easy-to-use prognostic model that may be used to risk stratify monitoring for LEF toxicity and to make informed choices about risks when choosing treatments.
Keywords: drug toxicity; leflunomide; monitoring; psoriatic arthritis; rheumatoid arthritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Smolen JS, Landewé RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. - PubMed
-
- Strand V, Cohen S, Schiff M. et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med 1999;159:2542–50. - PubMed
-
- Cohen S, Cannon GW, Schiff M. et al. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis Rheum 2001;44:1984–92. - PubMed
-
- Emery P, Breedveld FC, Lemmel EM. et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000;39:655–65. - PubMed

