A Randomized Trial of Point-of-Care Early Infant Human Immunodeficiency Virus (HIV) Diagnosis in Zambia
- PMID: 34718462
- PMCID: PMC9410723
- DOI: 10.1093/cid/ciab923
A Randomized Trial of Point-of-Care Early Infant Human Immunodeficiency Virus (HIV) Diagnosis in Zambia
Abstract
Background: Point-of-care (POC) early infant diagnosis (EID) provides same-day results and the potential for immediate initiation of antiretroviral therapy (ART).
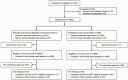
Methods: We conducted a pragmatic trial at 6 public clinics in Zambia. HIV-exposed infants were individually randomized to either (1) POC EID (onsite testing with the Alere q HIV-1/2 Detect) or (2) enhanced standard of care (SOC) EID (off-site testing at a public laboratory). Infants with HIV were referred for ART and followed for 12 months. Our primary outcome was defined as alive, in care, and virally suppressed at 12 months.
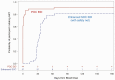
Results: Between March 2016 and November 2018, we randomized 4000 HIV-exposed infants to POC (n=1989) or SOC (n=2011). All but 2 infants in the POC group received same-day results, while the median time to result in the SOC group was 27 (interquartile range: 22-30) days. Eighty-one (2%; 95% confidence interval [CI]: 1.6-2.5%) infants were diagnosed with HIV. Although ART initiation was high, there were 15 (19%) deaths, 15 (19%) follow-up losses, and 31 (38%) virologic failures. By 12 months, only 20 of 81 (25%; 95% CI: 15-34%) infants with HIV were alive, in care, and virally suppressed: 13 (30%; 16-43%) infants in the POC group vs 7 (19%; 6-32%) in the SOC group (RR: 1.56; .7-3.50).
Conclusions: POC EID eliminated diagnostic delays and accelerated ART initiation but did not translate into definitive improvement in 12-month outcomes. In settings where centralized EID is well functioning, POC EID is unlikely to improve pediatric HIV outcomes.
Clinical trials registration: This trial is registered at https://clinicaltrials.gov (NCT02682810).
Keywords: early infant diagnosis of HIV; pediatric HIV low- and middle income country; point of care diagnosis; prevention of mother to child HIV transmission.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. S. A. S. reports support from the Bill and Melinda Gates Foundation, outside the submitted work. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDSinfo data sheet: pregnant women needed ART for PMTCT. 2019. Available at: https://aidsinfo.unaids.org. Accessed 15 December 2019. - PubMed
-
- UNAIDS. Start Free Stay Free AIDS Free: 2020 report. 2020. Available at: https://www.unaids.org/sites/default/files/media_asset/start-free-stay-f.... Accessed 16 November 2020.
-
- UNAIDS. Start Free Stay Free AIDS Free: 2019 report. 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/20190722_UNAIDS_S.... Accessed 15 December 2019.

