No Evidence of Long-Term Disruption of Glycometabolic Control After SARS-CoV-2 Infection
- PMID: 34718627
- PMCID: PMC8691144
- DOI: 10.1210/clinem/dgab792
No Evidence of Long-Term Disruption of Glycometabolic Control After SARS-CoV-2 Infection
Abstract
Purpose: To assess whether dysglycemia diagnosed during severe acute respiratory syndrome coronavirus 2 pneumonia may become a potential public health problem after resolution of the infection. In an adult cohort with suspected coronavirus disease 2019 (COVID-19) pneumonia, we integrated glucose data upon hospital admission with fasting blood glucose (FBG) in the year prior to COVID-19 and during postdischarge follow-up.
Methods: From February 25 to May 15, 2020, 660 adults with suspected COVID-19 pneumonia were admitted to the San Raffaele Hospital (Milan, Italy). Through structured interviews/ medical record reviews, we collected demographics, clinical features, and laboratory tests upon admission and additional data during hospitalization or after discharge and in the previous year. Upon admission, we classified participants according to American Diabetes Association criteria as having (1) preexisting diabetes, (2) newly diagnosed diabetes, (3) hyperglycemia not in the diabetes range, or (4) normoglycemia. FBG prior to admission and during follow-up were classified as normal or impaired fasting glucose and fasting glucose in the diabetes range.
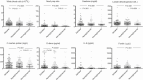
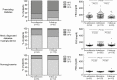
Results: In patients with confirmed COVID (n = 589), the proportion with preexisting or newly diagnosed diabetes, hyperglycemia not in the diabetes range and normoglycemia was 19.6%, 6.7%, 43.7%, and 30.0%, respectively. Patients with dysglycemia associated to COVID-19 had increased markers of inflammation and organs' injury and poorer clinical outcome compared to those with normoglycemia. After the infection resolved, the prevalence of dysglycemia reverted to preadmission frequency.
Conclusions: COVID-19-associated dysglycemia is unlikely to become a lasting public health problem. Alarmist claims on the diabetes risk after COVID-19 pneumonia should be interpreted with caution.
Keywords: COVID-19; diabetes; humans.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Figliozzi S, Masci PG, Ahmadi N, et al. . Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10):e13362. - PubMed
-
- Lee MH, Wong C, Ng CH, Yuen DC, Lim AY, Khoo CM. Effects of hyperglycaemia on complications of COVID-19: a meta-analysis of observational studies. Diabetes Obes. Metab. 2021;23(1):287-289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

