MR imaging characteristics of uveal melanoma with histopathological validation
- PMID: 34718831
- PMCID: PMC8724164
- DOI: 10.1007/s00234-021-02825-5
MR imaging characteristics of uveal melanoma with histopathological validation
Abstract
Purpose: To evaluate the magnetic resonance imaging (MRI) characteristics of uveal melanoma (UM), to compare them with fundoscopy and ultrasound (US), and to validate them with histopathology.
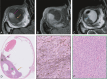
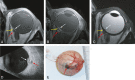
Methods: MR images from 42 UM were compared with US and fundoscopy, and on 14 enucleated cases with histopathology.
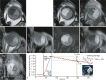
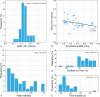
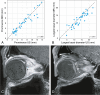
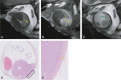
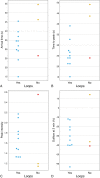
Results: A significant relationship between the signal intensity on T1 and pigmentation on histopathology was found (p=0.024). T1 hyperintense UM were always moderately or strongly pigmented on histopathology, while T1-hypointense UM were either pigmented or non-pigmented. Mean apparent diffusion coefficient (ADC) of the UM was 1.16 ± 0.26 × 10-3 mm2/s. Two-thirds of the UM had a wash-out and the remaining a plateau perfusion time-intensity curve (TIC). MRI was limited in evaluating the basal diameter of flat tumors. US tends to show larger tumor prominence (0.5mm larger, p=0.008) and largest basal diameter (1.4mm larger, p<0.001). MRI was good in diagnosing ciliary body involvement, extrascleral extension, and optic nerve invasion, but limited on identifying scleral invasion. An increase of tumor prominence was associated with lower ADC values (p=0.030) and favored a wash-out TIC (p=0.028). An increase of tumor ADC correlated with a plateau TIC (p=0.011).
Conclusions: The anatomical and functional MRI characteristics of UM were comprehensively assessed. Knowing the MRI characteristics of UM is important in order to confirm the diagnosis and to differentiate UM from other intra-ocular lesions and because it has implications for treatment planning. MRI is a good technique to evaluate UM, being only limited in case of flat tumors or on identifying scleral invasion.
Keywords: Histopathology; Magnetic resonance imaging; Ocular oncology; Ultrasound; Uveal melanoma.
© 2021. The Author(s).
Conflict of interest statement
We received research support from Philips Healthcare.
Figures


References
-
- Beenakker JWM, Ferreira TA, Soemarwoto KP, et al. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magn Reson Mater Physics, Biol Med. 2016;29:571–577. doi: 10.1007/s10334-016-0529-4. - DOI - PMC - PubMed
-
- Foti PV, Longo A, Reibaldi M et al (2017) Uveal melanoma: quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol Medica. 10.1007/s11547-016-0697-3 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

