Falls and Fractures in Patients with Parkinson's Disease-Related Psychosis Treated with Pimavanserin vs Atypical Antipsychotics: A Cohort Study
- PMID: 34718963
- PMCID: PMC8844331
- DOI: 10.1007/s40801-021-00284-1
Falls and Fractures in Patients with Parkinson's Disease-Related Psychosis Treated with Pimavanserin vs Atypical Antipsychotics: A Cohort Study
Abstract
Background: Parkinson's disease-related psychosis increases patients' risk of falls. Pimavanserin is an atypical antipsychotic approved in the USA in 2016 for the treatment of hallucinations and delusions associated with Parkinson's disease-related psychosis.
Objective: We aimed to compare the risk of falls/fractures among patients with Parkinson's disease-related psychosis treated with pimavanserin vs other atypical antipsychotics.
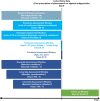
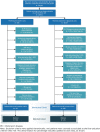
Patients and methods: We identified a cohort of patients with Parkinson's disease-related psychosis aged ≥ 40 years initiating either pimavanserin or a comparator antipsychotic (clozapine, quetiapine, risperidone, olanzapine, aripiprazole, brexpiprazole) in US commercial insurance and supplementary Medicare claims (2015-2019). Comparators were propensity score matched 2:1 with pimavanserin initiators; incidence rates of falls/fractures were compared using incidence rate ratios (IRRs) and 95% confidence intervals (CIs).
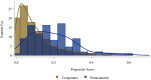
Results: We identified 112 eligible pimavanserin initiators and 982 comparators. Pimavanserin initiators were younger and had fewer severe comorbidities, indicators of impairment, and healthcare encounters, though they had higher Parkinson's disease medication use. The crude incidence rates [cases/100 person-years] (95% CI) for composite falls/fractures were 17.8 (7.7-35.0) for pimavanserin and 40.8 (35.0-47.4) for comparators. Matching retained 108 pimavanserin initiators and 216 comparators-all characteristics were well balanced after matching-with a matched IRR (pimavanserin vs comparator) of 0.71 (95% CI 0.27-1.67). Sensitivity analysis IRR estimates were consistently below 1.00, with a sensitivity analysis not requiring a diagnosis of psychosis resulting in an IRR estimate of 0.55 (95% CI 0.34-0.86).
Conclusions: The results of this study do not suggest an increase in the risk of falls or fractures associated with pimavanserin compared with other antipsychotics in patients with Parkinson's disease-related psychosis. Sensitivity analyses suggest a decreased risk.
© 2021. The Author(s).
Conflict of interest statement
CD and MET are employees of Acadia Pharmaceuticals, which produces one of the medications evaluated in this study. GD was an employee of Acadia Pharmaceuticals while this work was performed. JBL, JF, JLB, MSA, and HED are employees of RTI Health Solutions. MER was an employee of RTI Health Solutions while this work was performed. This study was conducted by RTI Health Solutions with funding from Acadia Pharmaceuticals under a contract that included independent publication rights.
Figures

References
-
- Nuplazid (pimavanserin) prescribing information. San Diego (CA): Acadia Pharmaceuticals Inc.; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207318s009,210.... Accessed 18 Nov 2019.
-
- U.S. Centers for Medicare & Medicaid Services. 2018 ICD-10 CM and GEMs. 11 August 2017, 2017. https://www.cms.gov/Medicare/Coding/ICD10/2018-ICD-10-CM-and-GEMs. Accessed 25 Aug 2021.
LinkOut - more resources
Full Text Sources

