Different epitopes of Ralstonia solanacearum effector RipAW are recognized by two Nicotiana species and trigger immune responses
- PMID: 34719088
- PMCID: PMC8743020
- DOI: 10.1111/mpp.13153
Different epitopes of Ralstonia solanacearum effector RipAW are recognized by two Nicotiana species and trigger immune responses
Erratum in
-
Correction to 'Different epitopes of Ralstonia solanacearum effector RipAW are recognized by two Nicotiana species and trigger immune responses'.Mol Plant Pathol. 2024 Jan;25(1):e13420. doi: 10.1111/mpp.13420. Mol Plant Pathol. 2024. PMID: 38279857 Free PMC article. No abstract available.
Abstract
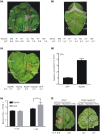
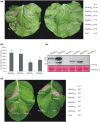
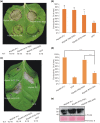
Diverse pathogen effectors convergently target conserved components in plant immunity guarded by intracellular nucleotide-binding domain leucine-rich repeat receptors (NLRs) and activate effector-triggered immunity (ETI), often causing cell death. Little is known of the differences underlying ETI in different plants triggered by the same effector. In this study, we demonstrated that effector RipAW triggers ETI on Nicotiana benthamiana and Nicotiana tabacum. Both the first 107 amino acids (N1-107 ) and RipAW E3-ligase activity are required but not sufficient for triggering ETI on N. benthamiana. However, on N. tabacum, the N1-107 fragment is essential and sufficient for inducing cell death. The first 60 amino acids of the protein are not essential for RipAW-triggered cell death on either N. benthamiana or N. tabacum. Furthermore, simultaneous mutation of both R75 and R78 disrupts RipAW-triggered ETI on N. tabacum, but not on N. benthamiana. In addition, N. tabacum recognizes more RipAW orthologs than N. benthamiana. These data showcase the commonalities and specificities of RipAW-activated ETI in two evolutionally related species, suggesting Nicotiana species have acquired different abilities to perceive RipAW and activate plant defences during plant-pathogen co-evolution.
Keywords: Nicotiana; Ralstonia solanacearum; E3 ligase; RipAW; cell death; effector; effector-triggered immunity.
© 2021 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures



References
-
- Angot, A. , Peeters, N. , Lechner, E. , Vailleau, F. , Baud, C. , Gentzbittel, L. et al. (2006) Ralstonia solanacearum requires F‐box‐like domain‐containing type III effectors to promote disease on several host plants. Proceedings of the National Academy of Sciences USA, 103, 14620–14625. - PMC - PubMed
-
- Azevedo, C. , Sadanandom, A. , Kitagawa, K. , Freialdenhoven, A. , Shirasu, K. & Schulze‐Lefert, P. (2002) The RAR1 interactor SGT1, an essential component of R gene‐triggered disease resistance. Science, 295, 2073–2076. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

