The effects of osteopathic manipulative treatment on pain and disability in patients with chronic neck pain: A single-blinded randomized controlled trial
- PMID: 34719122
- PMCID: PMC9054945
- DOI: 10.1002/pmrj.12732
The effects of osteopathic manipulative treatment on pain and disability in patients with chronic neck pain: A single-blinded randomized controlled trial
Abstract
Background: Neck pain (NP) affects up to 70% of individuals at some point in their lives. Systematic reviews indicate that manual treatments can be moderately effective in the management of chronic, nonspecific NP. However, there is a paucity of studies specifically evaluating the efficacy of osteopathic manipulative treatment (OMT).
Objective: To evaluate the efficacy of OMT in reducing pain and disability in patients with chronic NP.
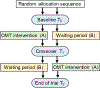
Design: Single-blinded, cross-over, randomized-controlled trial.
Setting: University-based, osteopathic manipulative medicine outpatient clinic.
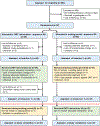
Participants: Ninety-seven participants, 21 to 65 years of age, with chronic, nonspecific NP.
Interventions: Participants were randomized to two trial arms: immediate OMT intervention or waiting period first. The intervention consisted of three to four OMT sessions over 4 to 6 weeks, after which the participants switched groups.
Main outcome measures: Primary outcome measures were pain intensity (average and current) on the numerical rating scale and Neck Disability Index. Secondary outcomes included Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29) health domains and Fear Avoidance Beliefs Questionnaire. Outcomes obtained prior to the cross-over allocation were evaluated using general linear models and after adjusting for baseline values.
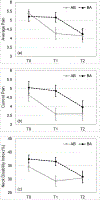
Results: A total of 38 and 37 participants were available for the analysis in the OMT and waiting period groups, respectively. The results showed significantly better primary outcomes in the immediate OMT group for reductions in average pain (-1.02, 95% confidence interval [CI] -1.72, -0.32; p = .005), current pain (-1.02, 95% CI -1.75, -0.30; p = .006), disability (-5.30%, 95% CI -9.2%, -1.3%; p = .010) and improved secondary outcomes (PROMIS) related to sleep (-3.25, 95% CI -6.95, -1.54; p = .003), fatigue (-3.26, 95% CI -6.04, -0.48; p = .022), and depression (-2.59, 95% CI -4.73, -0.45; p = .018). The effect sizes were in the clinically meaningful range between 0.5 and 1 standard deviation. No study-related serious adverse events were reported.
Conclusions: OMT is relatively safe and effective in reducing pain and disability along with improving sleep, fatigue, and depression in patients with chronic NP immediately following treatment delivered over approximately 4 to 6 weeks.
© 2021 American Academy of Physical Medicine and Rehabilitation.
Figures
References
-
- Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. Jul 2014;73(7):1309–1315. - PubMed
-
- Hurwitz EL, Randhawa K, Yu H, Cote P, Haldeman S. The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J Sep 2018;27(Suppl 6):796–801. - PubMed
-
- Cote P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine. Aug 1 1998;23(15):1689–1698. - PubMed
-
- Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). Feb 15 2008;33(4 Suppl):S39–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

