Protein tyrosine phosphatase receptor type O (PTPRO) knockdown enhances the proliferative, invasive and angiogenic activities of trophoblast cells by suppressing ER resident protein 44 (ERp44) expression in preeclampsia
- PMID: 34719307
- PMCID: PMC8810010
- DOI: 10.1080/21655979.2021.1997561
Protein tyrosine phosphatase receptor type O (PTPRO) knockdown enhances the proliferative, invasive and angiogenic activities of trophoblast cells by suppressing ER resident protein 44 (ERp44) expression in preeclampsia
Abstract
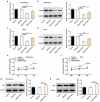
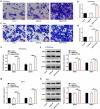
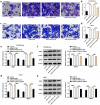
Preeclampsia (PE), a pregnancy-specific syndrome, is the primary cause of maternal mortality. This work was designed to investigate the specific functions of PTPRO/ ERp44 in the biological behaviors of trophoblast cells and elucidate the underlying molecular mechanism. Constructed siRNA-PTPRO and ERp44 overexpression plasmids were transfected into HTR-8/SVneo and JEG-3 cells for further functional experiments. Subsequently, the proliferation and invasion of trophoblast cells were identified by performing CCK-8, flow cytometry and transwell assay. In addition, tube formation assay was employed to estimate the angiogenic ability of HUVECs incubated with the conditioned media (CM) of HTR-8/SVneo or JEG-3 cells. Importantly, the interaction between PTPRO and ERp44 was analyzed through Co-IP. In the current investigation, it was discovered that downregulation of PTPRO notably facilitated the proliferation and invasion of trophoblast cells and induced a stronger in vitro angiogenesis. Moreover, PTPRO interacted with ERp44 to regulate ERp44 expression. ERp44 overexpression suppressed the proliferative, invasive and angiogenic activities of trophoblast cells. As a result, functions of PTPRO knockdown in the biological behaviors of trophoblast cells were partially abrogated upon elevation of ERp44. To sum up, this current research systematically evidenced that PTPRO could regulate the biological behaviors of trophoblast cells by modulating ERp44. Findings may contribute to a novel therapeutic strategy for PE.
Keywords: ERp44; PTPRO; preeclampsia; trophoblast cells.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Travaglino A, Raffone A, Saccone G, et al. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2019;234:200–206. - PubMed
-
- Hou J, Xu J, Jiang R, et al. Estrogen-sensitive PTPRO expression represses hepatocellular carcinoma progression by control of STAT3. Hepatology. 2013;57(2):678–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
