A prospective cohort study of confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy evaluating SARS-CoV-2 antibodies in maternal and umbilical cord blood and SARS-CoV-2 in vaginal swabs
- PMID: 34719780
- PMCID: PMC8653183
- DOI: 10.1111/aogs.14274
A prospective cohort study of confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy evaluating SARS-CoV-2 antibodies in maternal and umbilical cord blood and SARS-CoV-2 in vaginal swabs
Abstract
Introduction: Evidence about the consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnancy is rapidly increasing; however, data on antibody response and risk of transmission during pregnancy and delivery are still limited. The aim of this study was to evaluate if SARS-CoV-2 is detectable in vaginal swabs and whether antibodies against SARS-CoV-2 are present in maternal and umbilical cord blood of pregnant women with confirmed SARS-CoV-2.
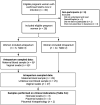
Material and methods: A single-unit prospective cohort study in Denmark including pregnant women with SARS-CoV-2 infection confirmed by a pharyngeal swab between August 20, 2020, and March 1, 2021, who gave birth during the same period. All patients admitted to the maternity ward and antepartum clinic were screened for SARS-CoV-2 infection. A maternal blood sample and vaginal swabs were collected at inclusion. If included antepartum, these samples were repeated intrapartum when an umbilical cord blood sample was also collected. Swabs were analyzed for SARS-CoV-2 and blood samples were analyzed for SARS-CoV-2 total antibodies. Placental and neonatal swabs as well as placental histopathological examinations were performed on clinical indications.
Results: We included 28 women, of whom four had serious maternal or fetal outcomes including one case of neonatal death. Within the first 8 days after confirmed SARS-CoV-2 infection, SARS-CoV-2 was detectable in two vaginal swabs (2/28) and SARS-CoV-2 antibodies were detected in 1 of 13 women. From 16 days after confirmed infection, antibodies were observed in 19 of 21 of women. Antibodies in cord blood were not detected during the first 16 days after confirmed infection (n = 7). However, from 26 days, antibodies were present in 16 of 17 cord blood samples of seropositive mothers. Placental examination in two cases of severe fetal outcomes preceded by reduced fetal movements revealed SARS-CoV-2 in swabs and severe histopathological abnormalities.
Conclusions: SARS-CoV-2 was detected in only 2 of 28 vaginal swabs within 8 days after confirmed infection in pregnant women. Our data suggest that maternal seroconversion occurs between days 8 and 16, whereas antibodies in cord blood of seropositive mothers were present in the majority from 26 days after confirmed infection. Additional data are needed regarding timing of seroconversion for the mother and appearance of antibodies in cord blood.
Keywords: cohort studies; coronavirus disease 2019; obstetric delivery; placental dysfunction; pregnancy complications; pregnancy outcome; prospective studies; severe acute respiratory syndrome coronavirus 2; vertical transmission.
© 2021 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
None.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

