Dietary citrate supplementation enhances longevity, metabolic health, and memory performance through promoting ketogenesis
- PMID: 34719871
- PMCID: PMC8672782
- DOI: 10.1111/acel.13510
Dietary citrate supplementation enhances longevity, metabolic health, and memory performance through promoting ketogenesis
Abstract
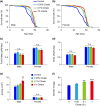
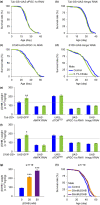
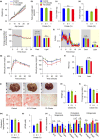
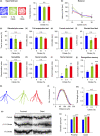
Citrate is an essential substrate for energy metabolism that plays critical roles in regulating cell growth and survival. However, the action of citrate in regulating metabolism, cognition, and aging at the organismal level remains poorly understood. Here, we report that dietary supplementation with citrate significantly reduces energy status and extends lifespan in Drosophila melanogaster. Our genetic studies in fruit flies implicate a molecular mechanism associated with AMP-activated protein kinase (AMPK), target of rapamycin (TOR), and ketogenesis. Mice fed a high-fat diet that supplemented with citrate or the ketone body β-hydroxybutyrate (βOHB) also display improved metabolic health and memory. These results suggest that dietary citrate supplementation may prove to be a useful intervention in the future treatment of age-related dysfunction.
Keywords: dendritic spine; hippocampus; insulin; lifespan.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Birkenfeld, A. L. , Lee, H.‐Y. , Guebre‐Egziabher, F. , Alves, T. C. , Jurczak, M. J. , Jornayvaz, F. R. , Zhang, D. , Hsiao, J. J. , Martin‐Montalvo, A. , Fischer‐Rosinsky, A. , Spranger, J. , Pfeiffer, A. F. , Jordan, J. , Fromm, M. F. , König, J. , Lieske, S. , Carmean, C. M. , Frederick, D. W. , Weismann, D. , … Shulman, G. I. (2011). Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metabolism, 14(2), 184–195. 10.1016/j.cmet.2011.06.009 - DOI - PMC - PubMed
-
- Chin, R. M. , Fu, X. , Pai, M. Y. , Vergnes, L. , Hwang, H. , Deng, G. , Diep, S. , Lomenick, B. , Meli, V. S. , Monsalve, G. C. , Hu, E. , Whelan, S. A. , Wang, J. X. , Jung, G. , Solis, G. M. , Fazlollahi, F. , Kaweeteerawat, C. , Quach, A. , Nili, M. , … Huang, J. (2014). The metabolite α‐ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature, 510(7505), 397–401. 10.1038/nature13264 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

