Long-Term Efficacy and Safety of Guselkumab, a Monoclonal Antibody Specific to the p19 Subunit of Interleukin-23, Through Two Years: Results From a Phase III, Randomized, Double-Blind, Placebo-Controlled Study Conducted in Biologic-Naive Patients With Active Psoriatic Arthritis
- PMID: 34719872
- PMCID: PMC9305108
- DOI: 10.1002/art.42010
Long-Term Efficacy and Safety of Guselkumab, a Monoclonal Antibody Specific to the p19 Subunit of Interleukin-23, Through Two Years: Results From a Phase III, Randomized, Double-Blind, Placebo-Controlled Study Conducted in Biologic-Naive Patients With Active Psoriatic Arthritis
Abstract
Objective: To assess long-term efficacy and safety of guselkumab, an interleukin-23 p19 subunit (IL-23p19) inhibitor, in patients with active psoriatic arthritis (PsA) from the phase III DISCOVER-2 trial.
Methods: In the DISCOVER-2 trial, patients with active PsA (≥5 swollen joints and ≥5 tender joints; C-reactive protein level ≥0.6 mg/dl) despite prior nonbiologic therapy were randomized to receive the following: guselkumab 100 mg every 4 weeks; guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks; or placebo with crossover to guselkumab 100 mg every 4 weeks, beginning at week 24. Efficacy assessments included American College of Rheumatology ≥20%/50%/70% improvement criteria (ACR20/50/70), Investigator's Global Assessment (IGA) of psoriasis score of 0 (indicating complete skin clearance), resolution of enthesitis (Leeds Enthesitis Index) and dactylitis (Dactylitis Severity Score), and changes in the Sharp/van der Heijde modified radiographic scores for PsA. Clinical data (imputed as no response/no change from baseline if missing) and observed radiographic data were summarized through week 100; safety assessments continued through week 112.
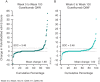
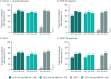
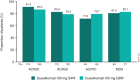
Results: Of the 739 randomized and treated patients, 652 (88%) completed treatment through week 100. Across groups of guselkumab-treated patients (including those in the placebo-guselkumab crossover group), the following findings at week 100 indicated that amelioration of arthritis signs/symptoms and extraarticular manifestations was durable through 2 years: ACR20 response (68-76%), ACR50 response (48-56%), ACR70 response (30-36%), IGA score of 0 (55-67%), enthesitis resolution (62-70%), and dactylitis resolution (72-83%). Mean changes in the Sharp/van der Heijde modified score for PsA from weeks 52 to week 100 (range 0.13-0.75) indicated that the low rates of radiographic progression observed among guselkumab-treated patients at earlier time points extended through week 100. Through week 112, 8% (5.8 per 100 patient-years) and 3% (1.9 per 100 patient-years) of the 731 guselkumab-treated patients had a serious adverse event or serious infection, respectively; 1 death occurred (road traffic accident).
Conclusion: In biologic-naive PsA patients, guselkumab provided durable improvements in multiple disease domains with no unexpected safety findings through 2 years.
© 2021 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta‐Felquer ML, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. - PubMed
-
- Mateo Soria L, Prior‐Espanol A, Grigorov MM, Holgado‐Perez S, Aparicio‐Espinar M, Martinez‐Morillo M, et al. Long‐term survival of biological therapy in psoriatic arthritis: 18‐year analysis of a cohort in a tertiary hospital. Rheumatol Int 2021. doi: 10.1007/s00296-021-04928-x. E‐pub ahead of print. - DOI - PubMed
-
- Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum 2017;47:29–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

