OptiDose: Computing the Individualized Optimal Drug Dosing Regimen Using Optimal Control
- PMID: 34720180
- PMCID: PMC8550736
- DOI: 10.1007/s10957-021-01819-w
OptiDose: Computing the Individualized Optimal Drug Dosing Regimen Using Optimal Control
Abstract
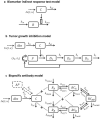
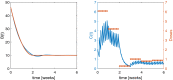
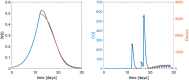
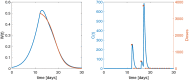
Providing the optimal dosing strategy of a drug for an individual patient is an important task in pharmaceutical sciences and daily clinical application. We developed and validated an optimal dosing algorithm (OptiDose) that computes the optimal individualized dosing regimen for pharmacokinetic-pharmacodynamic models in substantially different scenarios with various routes of administration by solving an optimal control problem. The aim is to compute a control that brings the underlying system as closely as possible to a desired reference function by minimizing a cost functional. In pharmacokinetic-pharmacodynamic modeling, the controls are the administered doses and the reference function can be the disease progression. Drug administration at certain time points provides a finite number of discrete controls, the drug doses, determining the drug concentration and its effect on the disease progression. Consequently, rewriting the cost functional gives a finite-dimensional optimal control problem depending only on the doses. Adjoint techniques allow to compute the gradient of the cost functional efficiently. This admits to solve the optimal control problem with robust algorithms such as quasi-Newton methods from finite-dimensional optimization. OptiDose is applied to three relevant but substantially different pharmacokinetic-pharmacodynamic examples.
Keywords: Individualized optimal drug dosing; Model predictive control; Optimal control; Pharmacokinetic–pharmacodynamic models; Quasi-Newton methods.
© The Author(s) 2021.
Figures
References
-
- Van Donge T, Evers K, Koch G, van den Anker J, Pfister M. Clinical Pharmacology and Pharmacometrics to Better Understand Physiological Changes During Pregnancy and Neonatal Life. Handbook of Experimental Pharmacology. Berlin: Springer; 2019. - PubMed
-
- Gibaldi M, Perrier S. Pharmacokinetics. Boca Raton: CRC Press Tayler & Francis Group; 1982.
LinkOut - more resources
Full Text Sources
Other Literature Sources
