Patient Preferences: Results of a German Adaptive Choice-Based Conjoint Analysis (Market Research Study Sponsored by Eli Lilly and Company) in Patients on Palliative Treatment for Advanced Breast Cancer
- PMID: 34720809
- PMCID: PMC8543321
- DOI: 10.1159/000513139
Patient Preferences: Results of a German Adaptive Choice-Based Conjoint Analysis (Market Research Study Sponsored by Eli Lilly and Company) in Patients on Palliative Treatment for Advanced Breast Cancer
Abstract
Introduction: Integration of patient preferences into shared decision making improves disease-related outcomes, but such data from patients with advanced breast cancer (aBC) are limited. The objective of this study was to demonstrate the relative importance of overall survival (OS) and progression-free survival (PFS) in relation to quality of life (QoL) and therapy-associated side effects from the perspective of patients with aBC.
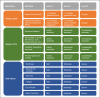
Methods: Postmenopausal patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative aBC receiving first- or second-line treatment were recruited throughout Germany. Patient-relevant attributes for aBC therapy assessment were collected using a stepwise multimodal approach. A conjoint matrix was developed, resulting in 2 attributes for therapy goals (OS and PFS), 4 for QoL, and 6 for side effects. An online quantitative survey was then performed using adaptive choice-based conjoint (ACBC) methodology.
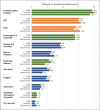
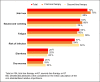
Results: The quantitative survey included 104 patients: 67 (64.4%) receiving first-line treatment and 37 (35.6%) receiving second-line treatment. The QoL attribute "physical agility and mobility" received the highest utility score (19.4 of 100%), reflecting the greatest importance to patients, followed by treatment goals (OS [15.2%] and PFS [14.4%]). Therapy-related side effects were less important, with nausea/vomiting being the most important (9.3%), followed by infection (6.4%) and hair loss (5.0%). The McFadden pseudo R2 (0.805), the root likelihood (0.864), and the χ2 test (2,809.041; p < 0.0001) indicated a very good fit of the statistical model.
Conclusion: Using ACBC analysis, it appears that QoL, OS, and PFS are most important to postmenopausal patients with aBC in relation to cancer treatment. Side effects seem to be less important if OS or PFS are prolonged and the QoL is maintained. Thus, QoL, OS, and PFS should be considered equally when making treatment decisions in aBC.
Keywords: Advanced breast cancer; Conjoint analysis; Patient preferences; Survival; Treatment.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
Dr. Reinisch has received honoraria from AstraZeneca, Eli Lilly and Company, MSD, Novartis, Pfizer, and Roche for advisory boards and/or lectures, and travel/accommodation expenses from Celgene, Novartis, and Pfizer. Dr. Marschner has nothing to disclose. Prof. Wöckel has received honoraria from Amgen, AstraZeneca, Aurikamed, Celgene, Eisai, Eli Lilly and Company, MSD, Novartis, Pfizer, Roche, Sirtex, and Tesaro for advisory boards and/or lectures. Thorsten Otto, Agnieszka Korfel, and Clemens Stoffregen are employees of Eli Lilly and Company.
Figures

References
-
- Reinert T, de Paula B, Shafaee MN, Souza PH, Ellis MJ, Bines J. Endocrine therapy for ER-positive/HER2-negative metastatic breast cancer. Linchuang Zhongliuxue Zazhi. 2018 Jun;7((3)):25. - PubMed
-
- van Til JA, Ijzerman MJ. Why should regulators consider using patient preferences in benefit-risk assessment? Pharmacoeconomics. 2014 Jan;32((1)):1–4. - PubMed
-
- Mühlbacher AC, Bethge S, Tockhorn A. Präferenzmessung im Gesundheitswesen: grundlagen von Discrete-Choice-Experimenten. Gesundheitsökonomie Qual. 2013;18((04)):159–72.
LinkOut - more resources
Full Text Sources
Research Materials

