Medial Temporal Lobe Subregional Atrophy in Aging and Alzheimer's Disease: A Longitudinal Study
- PMID: 34720998
- PMCID: PMC8554299
- DOI: 10.3389/fnagi.2021.750154
Medial Temporal Lobe Subregional Atrophy in Aging and Alzheimer's Disease: A Longitudinal Study
Abstract
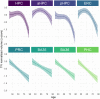
Medial temporal lobe (MTL) atrophy is a key feature of Alzheimer's disease (AD), however, it also occurs in typical aging. To enhance the clinical utility of this biomarker, we need to better understand the differential effects of age and AD by encompassing the full AD-continuum from cognitively unimpaired (CU) to dementia, including all MTL subregions with up-to-date approaches and using longitudinal designs to assess atrophy more sensitively. Age-related trajectories were estimated using the best-fitted polynomials in 209 CU adults (aged 19-85). Changes related to AD were investigated among amyloid-negative (Aβ-) (n = 46) and amyloid-positive (Aβ+) (n = 14) CU, Aβ+ patients with mild cognitive impairment (MCI) (n = 33) and AD (n = 31). Nineteen MCI-to-AD converters were also compared with 34 non-converters. Relationships with cognitive functioning were evaluated in 63 Aβ+ MCI and AD patients. All participants were followed up to 47 months. MTL subregions, namely, the anterior and posterior hippocampus (aHPC/pHPC), entorhinal cortex (ERC), Brodmann areas (BA) 35 and 36 [as perirhinal cortex (PRC) substructures], and parahippocampal cortex (PHC), were segmented from a T1-weighted MRI using a new longitudinal pipeline (LASHiS). Statistical analyses were performed using mixed models. Adult lifespan models highlighted both linear (PRC, BA35, BA36, PHC) and nonlinear (HPC, aHPC, pHPC, ERC) trajectories. Group comparisons showed reduced baseline volumes and steeper volume declines over time for most of the MTL subregions in Aβ+ MCI and AD patients compared to Aβ- CU, but no differences between Aβ- and Aβ+ CU or between Aβ+ MCI and AD patients (except in ERC). Over time, MCI-to-AD converters exhibited a greater volume decline than non-converters in HPC, aHPC, and pHPC. Most of the MTL subregions were related to episodic memory performances but not to executive functioning or speed processing. Overall, these results emphasize the benefits of studying MTL subregions to distinguish age-related changes from AD. Interestingly, MTL subregions are unequally vulnerable to aging, and those displaying non-linear age-trajectories, while not damaged in preclinical AD (Aβ+ CU), were particularly affected from the prodromal stage (Aβ+ MCI). This volume decline in hippocampal substructures might also provide information regarding the conversion from MCI to AD-dementia. All together, these findings provide new insights into MTL alterations, which are crucial for AD-biomarkers definition.
Keywords: Alzheimer's disease; aging; episodic memory; hippocampus; medial temporal lobe (MTL); mild cognitive impairment; structural magnetic resonance imaging.
Copyright © 2021 Chauveau, Kuhn, Palix, Felisatti, Ourry, de La Sayette, Chételat and de Flores.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Amlien I. K., Sneve M. H., Vidal-Piñeiro D., Walhovd K. B., Fjell A. M. (2018). The lifespan trajectory of the encoding-retrieval flip: a multimodal examination of medial parietal cortex contributions to episodic memory. J. Neurosci. 38, 8666–8679. 10.1523/JNEUROSCI.1702-17.2018 - DOI - PMC - PubMed
-
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. 10.18637/jss.v067.i01 - DOI
LinkOut - more resources
Full Text Sources

