Cholesterol Regulates Exosome Release in Cultured Astrocytes
- PMID: 34721384
- PMCID: PMC8551362
- DOI: 10.3389/fimmu.2021.722581
Cholesterol Regulates Exosome Release in Cultured Astrocytes
Abstract
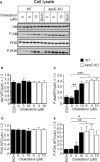
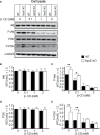
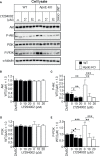
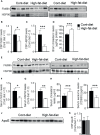
Exosomes are vesicles secreted by various kinds of cells, and they are rich in cholesterol, sphingomyelin (SM), phosphatidylcholine, and phosphatidylserine. Although cellular sphingolipid-mediated exosome release has been reported, the involvement of other lipid components of cell membranes in the regulation of exosome release is poorly understood. Here, we show that the level of exosome release into conditioned media is significantly reduced in cultured astrocytes prepared from apolipoprotein E (ApoE) knock-out mice when compared to those prepared from wild-type (WT) mice. The reduced level of exosome release was accompanied by elevated levels of cellular cholesterol. The addition of cholesterol to WT astrocytes significantly increased the cellular cholesterol levels and reduced exosome release. PI3K/Akt phosphorylation was enhanced in ApoE-deficient and cholesterol-treated WT astrocytes. In contrast, the depletion of cholesterol in ApoE-deficient astrocytes due to treatment with β-cyclodextrin recovered the exosome release level to a level similar to that in WT astrocytes. In addition, the reduced levels of exosome release due to the addition of cholesterol recovered to the control levels after treatment with a PI3K inhibitor (LY294002). The cholesterol-dependent regulation of exosome release was also confirmed by in vivo experiments; that is, exosome levels were significantly reduced in the CSF and blood serum of WT mice that were fed a high-fat diet and had increased cholesterol levels when compared to those in WT mice that were fed a normal diet. These results suggest that exosome release is regulated by cellular cholesterol via stimulation of the PI3K/Akt signal pathway.
Keywords: Akt; PI3K; apolipoprotein E; astrocytes; cholesterol; exosome.
Copyright © 2021 Abdullah, Nakamura, Ferdous, Gao, Chen, Zou and Michikawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

