The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation
- PMID: 34721436
- PMCID: PMC8551360
- DOI: 10.3389/fimmu.2021.765034
The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation
Abstract
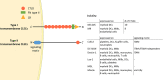
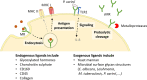
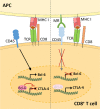
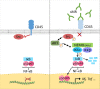
The mannose receptor is a member of the C-type lectin (CLEC) family, which can bind and internalize a variety of endogenous and pathogen-associated ligands. Because of these properties, its role in endocytosis as well as antigen processing and presentation has been studied intensively. Recently, it became clear that the mannose receptor can directly influence the activation of various immune cells. Cell-bound mannose receptor expressed by antigen-presenting cells was indeed shown to drive activated T cells towards a tolerogenic phenotype. On the other hand, serum concentrations of a soluble form of the mannose receptor have been reported to be increased in patients suffering from a variety of inflammatory diseases and to correlate with severity of disease. Interestingly, we recently demonstrated that the soluble mannose receptor directly promotes macrophage proinflammatory activation and trigger metaflammation. In this review, we highlight the role of the mannose receptor and other CLECs in regulating the activation of immune cells and in shaping inflammatory responses.
Keywords: biomarker; immunometabolism; macrophage; mannose receptor; metaflammation; sCD206; sMR.
Copyright © 2021 van der Zande, Nitsche, Schlautmann, Guigas and Burgdorf.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Shepherd VL, Abdolrasulnia R, Garrett M, Cowan HB. Down-Regulation of Mannose Receptor Activity in Macrophages After Treatment With Lipopolysaccharide and Phorbol Esters. J Immunol (1990) 145:1530–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

