AhABI4s Negatively Regulate Salt-Stress Response in Peanut
- PMID: 34721468
- PMCID: PMC8551806
- DOI: 10.3389/fpls.2021.741641
AhABI4s Negatively Regulate Salt-Stress Response in Peanut
Abstract
Soil salinity is one of the major factors that limit the area of cultivable land and yield potential of crops. The ability of salt tolerance varies with plant species. Peanut (Arachis hypogaea L.) is a moderately salt-sensitive and economically important crop, however, their biological processes involved in salt-stress response remain unclear. In this study, we investigated the role of A. hypogaea L. ABSCISIC ACID INSENSITIVE 4s (AhABI4s) in salt tolerance and elucidated its mode of action in peanuts. The results showed that the downregulation of AhABI4s via whole plant virus-induced gene silencing has enhanced the survival rate, biomass accumulation, and root/shoot ratio of peanut seedlings in response to salt-stress. Transcriptomics, quantitative proteomics, and phosphoproteomic analyses were performed using AhABI4s-silenced and Mock plants. The expression pattern of 15,247 genes, 1,900 proteins, and 2,620 phosphorylation sites were affected by silencing of AhABI4s in peanut leaf and root after sodium chloride (NaCl) treatment. Among them, 63 potential downstream target genes of ABI4 changed consistently at both transcription and translation levels, and the protein/phosphorylation levels of 31 ion transporters/channels were also affected. Electrophoretic mobility shift assays (EMSA) showed that ABI4 was able to bind to the promoters of HSP70, fructokinase (FRK), and pyruvate kinase (PK) coding genes in vitro. In addition, we also detected a binding preference of AhABI4 for CACT(G/T)GCA motif in the promoters of down-regulated genes in peanut leaf. Collectively, the potential downstream targets which were regulated at the levels of transcription and translation, binding preference, and in vivo phosphorylation sites that had been revealed in this study will provide new insight into the AhABI4s-mediated salt tolerance regulation mechanism in peanuts.
Keywords: downstream target; ion transporter/channel; peanut ABI4s; quantitative proteome and phosphoproteome; salt stress; transcriptome.
Copyright © 2021 Luo, Wan, Zhang, Zhang, Guo, Wang, Zheng, Liu, Ding and Wan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
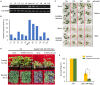

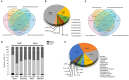

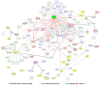
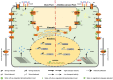
References
-
- Ayub M. A., Ahmad H. R., Ali M., Rizwan M., Ali S., Zia ur Rehman M., et al. . (2020). Salinity and its tolerance strategies in plants, in Plant Life Under Changing Environment, eds Tripathi D. K., Pratap Singh V., Chauhan D. K., Sharma S., Prasad S. M., Dubey N. K., Ramawat N. (Cambridge, MA: Academic Press; ), 47–76. 10.1016/B978-0-12-818204-8.00003-5 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

