Efficient Multi-Sites Genome Editing and Plant Regeneration via Somatic Embryogenesis in Picea glauca
- PMID: 34721480
- PMCID: PMC8551722
- DOI: 10.3389/fpls.2021.751891
Efficient Multi-Sites Genome Editing and Plant Regeneration via Somatic Embryogenesis in Picea glauca
Abstract
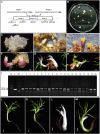
Conifers are the world's major source of timber and pulpwood and have great economic and ecological value. Currently, little research on the application of CRISPR/Cas9, the commonly used genome-editing tool in angiosperms, has been reported in coniferous species. An efficient CRISPR/Cas9 system based on somatic embryogenesis (SEis) suitable for conifers could benefit both fundamental and applied research in these species. In this study, the SpCas9 gene was optimized based on codon bias in white spruce, and a spruce U6 promoter was cloned and function-validated for use in a conifer specific CRISPR/Cas9 toolbox, i.e., PgCas9/PaU6. With this toolbox, a genome-editing vector was constructed to target the DXS1 gene of white spruce. By Agrobacterium-mediated transformation, the genome-editing vector was then transferred into embryogenic tissue of white spruce. Three resistant embryogenic tissues were obtained and used for regenerating plants via SEis. Albino somatic embryo (SE) plants with mutations in DXS1 were obtained in all of the three events, and the ratios of the homozygous and biallelic mutants in the 18 albino mutants detected were 22.2% in both cases. Green plants with mutations in DXS1 were also produced, and the ratios of the DXS1 mutants to the total green plants were 7.9, 28, and 13.5%, respectively, among the three events. Since 22.7% of the total 44 mutants were edited at both of the target sites 1 and 2, the CRISPR/Cas9 toolbox in this research could be used for multi-sites genome editing. More than 2,000 SE plants were regenerated in vitro after genome editing, and part of them showed differences in plant development. Both chimerism and mosaicism were found in the SE plants of white spruce after genome editing with the CRISPR/Cas9 toolbox. The conifer-specific CRISPR/Cas9 system developed in this research could be valuable in gene function research and trait improvement.
Keywords: CRISPR/Cas9; DXS1; Picea glauca; conifer; genome editing; gymnosperm; plant regeneration; somatic embryogenesis.
Copyright © 2021 Cui, Zhao, Gao, Zhao, Zhang and Kong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

