Maize Transformation: From Plant Material to the Release of Genetically Modified and Edited Varieties
- PMID: 34721493
- PMCID: PMC8553389
- DOI: 10.3389/fpls.2021.766702
Maize Transformation: From Plant Material to the Release of Genetically Modified and Edited Varieties
Abstract
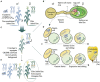
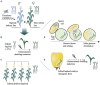
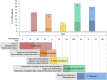
Over the past decades, advances in plant biotechnology have allowed the development of genetically modified maize varieties that have significantly impacted agricultural management and improved the grain yield worldwide. To date, genetically modified varieties represent 30% of the world's maize cultivated area and incorporate traits such as herbicide, insect and disease resistance, abiotic stress tolerance, high yield, and improved nutritional quality. Maize transformation, which is a prerequisite for genetically modified maize development, is no longer a major bottleneck. Protocols using morphogenic regulators have evolved significantly towards increasing transformation frequency and genotype independence. Emerging technologies using either stable or transient expression and tissue culture-independent methods, such as direct genome editing using RNA-guided endonuclease system as an in vivo desired-target mutator, simultaneous double haploid production and editing/haploid-inducer-mediated genome editing, and pollen transformation, are expected to lead significant progress in maize biotechnology. This review summarises the significant advances in maize transformation protocols, technologies, and applications and discusses the current status, including a pipeline for trait development and regulatory issues related to current and future genetically modified and genetically edited maize varieties.
Keywords: gene editing; genetic modification; maize; morphogenic regulator-mediated transformation; plant biotechnology; plant transformation.
Copyright © 2021 Yassitepe, da Silva, Hernandes-Lopes, Dante, Gerhardt, Fernandes, Da Silva, Vieira, Bonatti and Arruda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

