A Systematic Review of Transcriptional Dysregulation in Huntington's Disease Studied by RNA Sequencing
- PMID: 34721539
- PMCID: PMC8554124
- DOI: 10.3389/fgene.2021.751033
A Systematic Review of Transcriptional Dysregulation in Huntington's Disease Studied by RNA Sequencing
Abstract
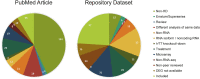
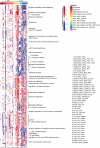
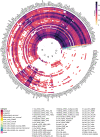
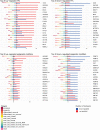
Huntington's disease (HD) is a chronic neurodegenerative disorder caused by an expansion of polyglutamine repeats in exon 1 of the Huntingtin gene. Transcriptional dysregulation accompanied by epigenetic alterations is an early and central disease mechanism in HD yet, the exact mechanisms and regulators, and their associated gene expression programs remain incompletely understood. This systematic review investigates genome-wide transcriptional studies that were conducted using RNA sequencing (RNA-seq) technology in HD patients and models. The review protocol was registered at the Open Science Framework (OSF). The biomedical literature and gene expression databases, PubMed and NCBI BioProject, Array Express, European Nucleotide Archive (ENA), European Genome-Phenome Archive (EGA), respectively, were searched using the defined terms specified in the protocol following the PRISMA guidelines. We conducted a complete literature and database search to retrieve all RNA-seq-based gene expression studies in HD published until August 2020, retrieving 288 articles and 237 datasets from PubMed and the databases, respectively. A total of 27 studies meeting the eligibility criteria were included in this review. Collectively, comparative analysis of the datasets revealed frequent genes that are consistently dysregulated in HD. In postmortem brains from HD patients, DNAJB1, HSPA1B and HSPB1 genes were commonly upregulated across all brain regions and cell types except for medium spiny neurons (MSNs) at symptomatic disease stage, and HSPH1 and SAT1 genes were altered in expression in all symptomatic brain datasets, indicating early and sustained changes in the expression of genes related to heat shock response as well as response to misfolded proteins. Specifically in indirect pathway medium spiny neurons (iMSNs), mitochondria related genes were among the top uniquely dysregulated genes. Interestingly, blood from HD patients showed commonly differentially expressed genes with a number of brain regions and cells, with the highest number of overlapping genes with MSNs and BA9 region at symptomatic stage. We also found the differential expression and predicted altered activity of a set of transcription factors and epigenetic regulators, including BCL6, EGR1, FOSL2 and CREBBP, HDAC1, KDM4C, respectively, which may underlie the observed transcriptional changes in HD. Altogether, our work provides a complete overview of the transcriptional studies in HD, and by data synthesis, reveals a number of common and unique gene expression and regulatory changes across different cell and tissue types in HD. These changes could elucidate new insights into molecular mechanisms of differential vulnerability in HD. Systematic Review Registration: https://osf.io/pm3wq.
Keywords: RNA sequencing; epigenetic regulators; huntington’s disease; transcription factors; transcriptional dysregulation.
Copyright © 2021 Malla, Guo, Senger, Chasapopoulou and Yildirim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Anderson K. E., van Duijn E., Craufurd D., Drazinic C., Edmondson M., Goodman N., et al. (2018). Clinical Management of Neuropsychiatric Symptoms of Huntington Disease: Expert-Based Consensus Guidelines on Agitation, Anxiety, Apathy, Psychosis and Sleep Disorders. J. Huntingtons Dis. 7, 355–366. 10.3233/jhd-180293 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

