Leishmania Parasites Differently Regulate Antioxidant Genes in Macrophages Derived From Resistant and Susceptible Mice
- PMID: 34722338
- PMCID: PMC8554229
- DOI: 10.3389/fcimb.2021.748738
Leishmania Parasites Differently Regulate Antioxidant Genes in Macrophages Derived From Resistant and Susceptible Mice
Abstract
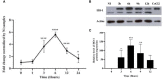
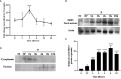
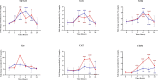
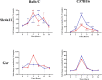
Macrophage-Leishmania interactions are central to parasite growth and disease outcome. Macrophages have developed various strategies to fight invaders, including oxidative burst. While some microorganisms seem to survive and even thrive in an oxidative environment, others are susceptible and get killed. To counter oxidative stress, macrophages switch the expressions of cytoprotective and detoxifying enzymes, which are downstream targets of the nuclear factor erythroid 2-related factor 2 (Nrf2), to enhance cell survival. We have explored the transcription of NRF2 and of its target genes and compared the effect of the parasite on their transcription in bone marrow-derived macrophages (BMdMs) from Leishmania-resistant and Leishmania-susceptible mice. While heme oxygenase 1 (HO-1) transcription is independent of the genetic background, the transcription of glutathione reductase (Gsr) and of cysteine/glutamate exchange transporter (Slc7a11), involved in glutathione accumulation, was differentially regulated in BMdMs from both mouse strains. We also show that, except for HO-1, known to favor the survival of the parasite, the transcription of the selected genes, including Gsr, CD36, and catalase (CAT), was actively repressed, if not at all time points at least at the later ones, by the parasite, especially in Balb/c BMdMs. Consistent with these results, we found that the silencing of NRF2 in this study increases the survival and multiplication of the parasite.
Keywords: Leishmania; NRF2; antioxidants; macrophage; resistance; susceptibility.
Copyright © 2021 Bichiou, Rabhi, Ben Hamda, Bouabid, Belghith, Piquemal, Trentin, Rabhi and Guizani-Tabbane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Araujo E. C. B., Barbosa B. F., Coutinho L. B., Barenco P. V. C., Sousa L. A., Milanezi C. M., et al. . (2013). Heme Oxygenase-1 Activity Is Involved in the Control of Toxoplasma Gondii Infection in the Lung of BALB/c and C57BL/6 and in the Small Intestine of C57BL/6 Mice. Vet. Res. 44, 89. doi: 10.1186/1297-9716-44-89 - DOI - PMC - PubMed
-
- Buchmüller-Rouiller Y., Corrandin S. B., Smith J., Schneider P., Ransijn A., Jongeneel C. V., et al. . (1995). Role of Glutathione in Macrophage Activation: Effect of Cellular Glutathione Depletion on Nitrite Production and Leishmanicidal Activity. Cell Immunol. 164, 73–80. doi: 10.1006/cimm.1995.1144 - DOI - PubMed
-
- Campbell N. K., Williams D. G., Fitzgerald H. K., Barry P. J., Cunningham C. C., Nolan D. P., et al. . (2019). Trypanosoma Brucei Secreted Aromatic Ketoacids Activate the Nrf2/HO-1 Pathway and Suppress Pro-Inflammatory Responses in Primary Murine Glia and Macrophages. Front. Immunol. 10, 2137. doi: 10.3389/fimmu.2019.02137 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

