Wnt1 Role in the Development of the Habenula and the Fasciculus Retroflexus
- PMID: 34722541
- PMCID: PMC8551717
- DOI: 10.3389/fcell.2021.755729
Wnt1 Role in the Development of the Habenula and the Fasciculus Retroflexus
Abstract
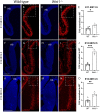
Wnt1 is one of the morphogenes that controls the specification and differentiation of neuronal populations in the developing central nervous system. The habenula is a diencephalic neuronal complex located in the most dorsal aspect of the thalamic prosomere. This diencephalic neuronal population is involved in the limbic system and its malfunction is related with several psychiatric disorders. Our aim is to elucidate the Wnt1 role in the habenula and its main efferent tract, the fasciculus retroflexus, development. In order to achieve these objectives, we analyzed these structures development in a Wnt1 lack of function mouse model. The habenula was generated in our model, but it presented an enlarged volume. This alteration was due to an increment in habenular neuroblasts proliferation rate. The fasciculus retroflexus also presented a wider and disorganized distribution and a disturbed final trajectory toward its target. The mid-hindbrain territories that the tract must cross were miss-differentiated in our model. The specification of the habenula is Wnt1 independent. Nevertheless, it controls its precursors proliferation rate. Wnt1 expressed in the isthmic organizer is vital to induce the midbrain and rostral hindbrain territories. The alteration of these areas is responsible for the fasciculus retroflexus axons misroute.
Keywords: Wnt1; differentiation; fasciculus retroflexus; habenula; proliferation.
Copyright © 2021 Company, Moreno-Cerdá, Andreu-Cervera, Murcia-Ramón, Almagro-García, Echevarría, Martínez and Puelles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Barth K. A., Kishimoto Y., Rohr K. B., Seydler C., Schulte-Merker S., Wilson S. W. (1999). Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development 126 4977–4987. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

