Antimicrobial and Antioxidant Secondary Metabolites from Trifolium baccarinii Chiov. (Fabaceae) and Their Mechanisms of Antibacterial Action
- PMID: 34722760
- PMCID: PMC8556085
- DOI: 10.1155/2021/3099428
Antimicrobial and Antioxidant Secondary Metabolites from Trifolium baccarinii Chiov. (Fabaceae) and Their Mechanisms of Antibacterial Action
Abstract
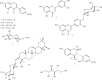
The treatment of infectious diseases with antimicrobial agents continues to present problems in modern-day medicine with many studies showing significant increase in the incidence of bacterial resistance to several antibiotics. The screening of antimicrobial activity of plant extracts and natural products has shown that medicinal plants are made up of a potential source of new anti-infective agents. The aim of this study was to evaluate the antimicrobial and antioxidant activities of extracts and compounds from the whole plant Trifolium baccarinii Chiov. and to determine their modes of antibacterial action. The plant extracts were prepared by maceration in organic solvents. The antimicrobial activities were evaluated using the broth microdilution method. The antioxidant activity was evaluated using the 2,2'-diphenyl-1-picrylhydrazyl radical (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) assays. The mechanisms of antibacterial action were determined by lysis, salt tolerance assays, and antioxidant enzyme activities. The cytotoxic effect on the erythrocytes was determined by a spectrophotometric method. Biochanin A, formononetin, luteolin, luteolin-4'-O-β-D-glucopyranoside, 4,7,2'-trihydroxy-4'-methoxyisoflavanol, sissotrin, 1-methyl-β-D-glucopyranoside, ononin, D-mannitol, and 3-O-β-D-glucuronopyranosylsoyasapogenol B were isolated from Trifolium baccarinii. The MeOH, EtOAc, and n-BuOH extracts as well as biochanin A, formononetin, luteolin, luteolin-4'-O-β-D-glucopyranoside, 4,7,2'-trihydroxy-4'-methoxyisoflavanol, and sissotrin from Trifolium baccarinii displayed the highest antimicrobial and antioxidant activities. The MeOH extract and 4,7,2'-trihydroxy-4'-methoxyisoflavanol exhibited antibacterial activity through the bacteriolytic effect and reduction of the antioxidant defenses in the bacterial cells. The present study portrays Trifolium baccarinii as a potential natural source of antibacterial, antifungal, and antioxidant agents.
Copyright © 2021 Donald Léonel Feugap Tsamo et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- World Health Organization (WHO) Antimicrobial Resistance Global Report on Surveillance . Geneva: WHO Press; 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

