Unconventional Disorder by Femtosecond Laser Irradiation in Fe2O3
- PMID: 34723005
- PMCID: PMC8552326
- DOI: 10.1021/acsomega.1c04079
Unconventional Disorder by Femtosecond Laser Irradiation in Fe2O3
Abstract
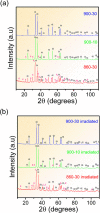
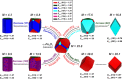
This paper demonstrates that femtosecond laser-irradiated Fe2O3 materials containing a mixture of α-Fe2O3 and ε-Fe2O3 phases showed significant improvement in their photoelectrochemical performance and magnetic and optical properties. The absence of Raman-active vibrational modes in the irradiated samples and the changes in charge carrier emission observed in the photocurrent density results indicate an increase in the density of defects and distortions in the crystalline lattice when compared to the nonirradiated ones. The magnetization measurements at room temperature for the nonirradiated samples revealed a weak ferromagnetic behavior, whereas the irradiated samples exhibited a strong one. The optical properties showed a reduction in the band gap energy and a higher conductivity for the irradiated materials, causing a higher current density. Due to the high performance observed, it can be applied in dye-sensitized solar cells and water splitting processes. Quantum mechanical calculations based on density functional theory are in accordance with the experimental results, contributing to the elucidation of the changes caused by femtosecond laser irradiation at the molecular level, evaluating structural, energetic, and vibrational frequency parameters. The surface simulations enable the construction of a diagram that elucidates the changes in nanoparticle morphologies.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Machala L.; Tuček J.; Zbořil R. Polymorphous transformations of nanometric iron ( III ) oxide: A review. Chem. Mater. 2011, 23, 3255–3272. 10.1021/cm200397g. - DOI
-
- Tronc E.; Chanéac C.; Jolivet J. P. Structural and magnetic characterization of e-Fe2O3. J. Solid State Chem. 1998, 139, 93–104. 10.1006/jssc.1998.7817. - DOI
-
- Petersen N.; Schembera N.; Schmidbauer E.; Vali H. Magnetization, mössbauer spectroscopy and structural studies of a ferrimagnetic Fe-Oxide formed by heating nontronite in air. Phys. Chem. Miner. 1987, 14, 118–121. 10.1007/bf00308215. - DOI
-
- McClean R. G.; Schofield M. A.; Kean W. F.; Sommer C. V.; Robertson D. P.; Toth D.; Gajdardziska-Josifovska M. Botanical iron minerals: Correlation between nanocrystal structure and modes of biological self-assembly. Eur. J. Mineral. 2001, 13, 1235–1242. 10.1127/0935-1221/2001/0013-1235. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

