Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses
- PMID: 34723209
- PMCID: PMC8547803
- DOI: 10.1016/S2665-9913(21)00328-3
Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses
Conflict of interest statement
KS contributed to project administration, investigation, formal analysis, visualisation, and review and editing of the report. NV and SP were involved in investigation, formal analysis, and development of methodology. BL contributed to conceptualisation, investigation, and formal analysis. WCA was involved in investigation and development of methodology. JvK contributed to conceptualisation, investigation, and review and editing of the report. AR-R was the project lead, and was involved in conceptualisation, writing the original draft of the report, investigation, and formal analysis. KS reports support for travel or meeting attendance from AbbVie Germany. WCA reports grants from the Gottfried & Julia Bangerter-Rhyner Stiftung, FUNGINOS, and the Swiss National Science Foundation; an internal grant from Kantonsspital St Gallen; honoraria from Pfizer and Medscape; and compensation for participation on a data safety monitoring board from Merck Sharp & Dohme and Sanofi. JvK reports honoraria from AbbVie, Bristol Myers Squibb (BMS), Boehringer-lngelheim, GlaxoSmithKline, Menarini, Novartis, Pfizer, and Sanofi. AR-R reports consulting fees from AbbVie, Gilead, Lilly, BMS, and Sanofi; honoraria from AbbVie, Pfizer, Sanofi, UCB, BMS, Lilly, Gilead, and Roche; payment for expert testimony from AbbVie and Gilead; support for travel or meeting attendance from Sanofi, Roche, and AbbVie; and compensation for participation on a data safety monitoring board from R-Pharm. All other authors declare no competing interests.
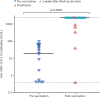
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

