Photoresponsive Control of G-Quadruplex DNA Systems
- PMID: 34723256
- PMCID: PMC8549047
- DOI: 10.1021/jacsau.1c00283
Photoresponsive Control of G-Quadruplex DNA Systems
Abstract
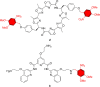
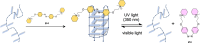
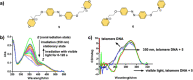
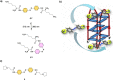
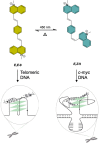
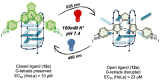
G-quadruplex (G4) oligonucleotide secondary structures have recently attracted significant attention as therapeutic targets owing to their occurrence in human oncogene promoter sequences and the genome of pathogenic organisms. G4s also demonstrate interesting catalytic activities in their own right, as well as the ability to act as scaffolds for the development of DNA-based materials and nanodevices. Owing to this diverse range of opportunities to exploit G4 in a variety of applications, several strategies to control G4 structure and function have emerged. Interrogating the role of G4s in biology requires the delivery of small-molecule ligands that promote its formation under physiological conditions, while exploiting G4 in the development of responsive nanodevices is normally achieved by the addition and sequestration of the metal ions required for the stabilization of the folded structure. Although these strategies prove successful, neither allows the system in question to be controlled externally. Meanwhile, light has proven to be an attractive means for the control of DNA-based systems as it is noninvasive, can be delivered with high spatiotemporal precision, and is orthogonal to many chemical and biological processes. A plethora of photoresponsive DNA systems have been reported to date; however, the vast majority deploy photoreactive moieties to control the stability and assembly of duplex DNA hybrids. Despite the unique opportunities afforded by the regulation of G-quadruplex formation in biology, catalysis, and nanotechnology, comparatively little attention has been devoted to the design of photoresponsive G4-based systems. In this Perspective, we consider the potential of photoresponsive G4 assemblies and examine the strategies that may be used to engineer these systems toward a variety of applications. Through an overview of the main developments in the field to date, we highlight recent progress made toward this exciting goal and the emerging opportunities that remain ripe for further exploration in the coming years.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


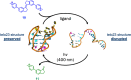
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
