Multifunctional Catalyst Combination for the Direct Conversion of CO2 to Propane
- PMID: 34723275
- PMCID: PMC8549042
- DOI: 10.1021/jacsau.1c00302
Multifunctional Catalyst Combination for the Direct Conversion of CO2 to Propane
Abstract
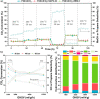
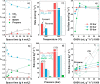
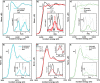
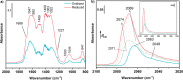
The production of carbon-rich hydrocarbons via CO2 valorization is essential for the transition to renewable, non-fossil-fuel-based energy sources. However, most of the recent works in the state of the art are devoted to the formation of olefins and aromatics, ignoring the rest of the hydrocarbon commodities that, like propane, are essential to our economy. Hence, in this work, we have developed a highly active and selective PdZn/ZrO2+SAPO-34 multifunctional catalyst for the direct conversion of CO2 to propane. Our multifunctional system displays a total selectivity to propane higher than 50% (with 20% CO, 6% C1, 13% C2, 10% C4, and 1% C5) and a CO2 conversion close to 40% at 350 °C, 50 bar, and 1500 mL g-1 h-1. We attribute these results to the synergy between the intimately mixed PdZn/ZrO2 and SAPO-34 components that shifts the overall reaction equilibrium, boosting CO2 conversion and minimizing CO selectivity. Comparison to a PdZn/ZrO2+ZSM-5 system showed that propane selectivity is further boosted by the topology of SAPO-34. The presence of Pd in the catalyst drives paraffin production via hydrogenation, with more than 99.9% of the products being saturated hydrocarbons, offering very important advantages for the purification of the products.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
From Lab to Technical CO2 Hydrogenation Catalysts: Understanding PdZn Decomposition.ACS Appl Mater Interfaces. 2023 Feb 1;15(4):5218-5228. doi: 10.1021/acsami.2c19357. Epub 2023 Jan 23. ACS Appl Mater Interfaces. 2023. PMID: 36688511 Free PMC article.
-
Identification of C2-C5 products from CO2 hydrogenation over PdZn/TiO2-ZSM-5 hybrid catalysts.Faraday Discuss. 2021 Jul 16;230(0):52-67. doi: 10.1039/d0fd00135j. Faraday Discuss. 2021. PMID: 33870391
-
Selectivity Control by Relay Catalysis in CO and CO2 Hydrogenation to Multicarbon Compounds.Acc Chem Res. 2024 Mar 5;57(5):714-725. doi: 10.1021/acs.accounts.3c00734. Epub 2024 Feb 13. Acc Chem Res. 2024. PMID: 38349801
-
New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels.Chem Soc Rev. 2019 Jun 17;48(12):3193-3228. doi: 10.1039/c8cs00502h. Chem Soc Rev. 2019. PMID: 31106785 Review.
-
Challenges and prospects in the selective photoreduction of CO2 to C1 and C2 products with nanostructured materials: a review.Mater Horiz. 2022 Feb 7;9(2):607-639. doi: 10.1039/d1mh01490k. Mater Horiz. 2022. PMID: 34897343 Review.
Cited by
-
The Oxygenate-Mediated Conversion of COx to Hydrocarbons─On the Role of Zeolites in Tandem Catalysis.Chem Rev. 2023 Oct 25;123(20):11775-11816. doi: 10.1021/acs.chemrev.3c00058. Epub 2023 Sep 28. Chem Rev. 2023. PMID: 37769023 Free PMC article. Review.
-
Atomically synergistic Zn-Cr catalyst for iso-stoichiometric co-conversion of ethane and CO2 to ethylene and CO.Nat Commun. 2024 Jan 30;15(1):911. doi: 10.1038/s41467-024-44918-8. Nat Commun. 2024. PMID: 38291043 Free PMC article.
-
Transitioning from Methanol to Olefins (MTO) toward a Tandem CO2 Hydrogenation Process: On the Role and Fate of Heteroatoms (Mg, Si) in MAPO-18 Zeotypes.JACS Au. 2024 Feb 13;4(2):744-759. doi: 10.1021/jacsau.3c00768. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425934 Free PMC article.
-
From Lab to Technical CO2 Hydrogenation Catalysts: Understanding PdZn Decomposition.ACS Appl Mater Interfaces. 2023 Feb 1;15(4):5218-5228. doi: 10.1021/acsami.2c19357. Epub 2023 Jan 23. ACS Appl Mater Interfaces. 2023. PMID: 36688511 Free PMC article.
-
In situ photodeposition of ultra-small palladium particles on TiO2.J Synchrotron Radiat. 2024 Sep 1;31(Pt 5):1071-1077. doi: 10.1107/S1600577524004788. Epub 2024 Jul 15. J Synchrotron Radiat. 2024. PMID: 39007821 Free PMC article.
References
-
- Markewitz P.; Kuckshinrichs W.; Leitner W.; Linssen J.; Zapp P.; Bongartz R.; Schreiber A.; Müller T. E. Worldwide Innovations in the Development of Carbon Capture Technologies and the Utilization of CO2. Energy Environ. Sci. 2012, 5 (6), 7281.10.1039/c2ee03403d. - DOI
-
- Centi G.; Quadrelli E. A.; Perathoner S. Catalysis for CO2 Conversion: A Key Technology for Rapid Introduction of Renewable Energy in the Value Chain of Chemical Industries. Energy Environ. Sci. 2013, 6 (6), 1711.10.1039/c3ee00056g. - DOI
-
- Ojelade O. A.; Zaman S. F. A Review on CO2 hydrogenation to Lower Olefins: Understanding the Structure-Property Relationships in Heterogeneous Catalytic Systems. Journal of CO2 Utilization 2021, 47, 101506.10.1016/j.jcou.2021.101506. - DOI
-
- Zhou W.; Cheng K.; Kang J.; Zhou C.; Subramanian V.; Zhang Q.; Wang Y. New Horizon in C1 Chemistry: Breaking the Selectivity Limitation in Transformation of Syngas and Hydrogenation of CO2 into Hydrocarbon Chemicals and Fuels. Chem. Soc. Rev. 2019, 48 (12), 3193–3228. 10.1039/C8CS00502H. - DOI - PubMed
