Antiepileptic drugs for seizure control in people with neurocysticercosis
- PMID: 34723391
- PMCID: PMC8559146
- DOI: 10.1002/14651858.CD009027.pub4
Antiepileptic drugs for seizure control in people with neurocysticercosis
Abstract
Background: Neurocysticercosis is the most common parasitic infection of the brain. Epilepsy is the most common clinical presentation, though people may also present with headache, symptoms of raised intracranial pressure, hydrocephalus, and ocular symptoms depending upon the localisation of the parasitic cysts. Anthelmintic drugs, antiepileptic drugs (AEDs), and anti-oedema drugs, such as steroids, form the mainstay of treatment. This is an updated version of the Cochrane Review previously published in 2019.
Objectives: To assess the effects (benefits and harms) of AEDs for the primary and secondary prevention of seizures in people with neurocysticercosis. For the question of primary prevention, we examined whether AEDs reduce the likelihood of seizures in people who had neurocysticercosis but had not had a seizure. For the question of secondary prevention, we examined whether AEDs reduce the likelihood of further seizures in people who had had at least one seizure due to neurocysticercosis. As part of primary prevention studies, we also aimed to examine which AED was beneficial in people with neurocysticercosis in terms of duration, dose, and side-effect profile.
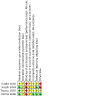
Search methods: For the 2021 update of this review, we searched the Cochrane Register of Studies (CRS Web), MEDLINE, and LILACS to January 2021. CRS Web includes randomised or quasi-randomised, controlled trials from CENTRAL, the Specialised Registers of Cochrane Review Groups, including Epilepsy, PubMed, Embase, ClinicalTrials.gov, and the World Health Organisation International Clinical Trials Registry Platform. We also checked the reference lists of identified studies, and contacted experts and colleagues in the field to search for additional and ongoing studies.
Selection criteria: Randomised and quasi-randomised controlled trials. Single-blind, double-blind, or unblinded studies were eligible for inclusion.
Data collection and analysis: We followed standard methodological procedures expected by Cochrane. Two review authors independently selected trials for inclusion and extracted the relevant data. The primary outcomes of interest were: proportion of individuals experiencing seizures, and time to first seizure post randomisation. Secondary outcomes included: seizure freedom, number of withdrawals, side effects, number of people seizure free with short or long durations of treatment, quality of life, therapy costs, hospitalisations, and mortality. We used an intention-to-treat analysis for the primary analysis. We calculated odds ratio (OR) for dichotomous data (proportion of individuals who experienced seizures, were seizure free for a specific time period (12 or 24 months), withdrew from treatment, developed drug-related side effects or complications, were seizure-free with each treatment policy, mortality), and planned to use mean difference (MD) for continuous data, if any continuous data were identified (quality of life, cost of treatment). We intended to evaluate time to first seizure after randomisation by calculating hazard ratios (HRs). We assessed precision using 95% confidence intervals (CIs). We stratified the analysis by treatment comparison. We also considered the duration of drug usage, co-medications, and the length of follow-up.
Main results: We did not find any trials that investigated the role of AEDs in preventing seizures among people with neurocysticercosis, presenting with symptoms other than seizures. We did not find any trials that directly compared individual AEDs for primary prevention in people with neurocysticercosis. We included four trials that evaluated the efficacy of short-term versus longer-term AED treatment for people with solitary neurocysticercosis (identified on computed tomography (CT) scan) who presented with seizures. In total, 466 people were enrolled. These studies compared AED treatment durations of 6, 12, and 24 months. The risk of seizure recurrence with six months of treatment compared with 12 to 24 months of treatment was inconclusive (odds ratio (OR) 1.34, 95% confidence interval (CI) 0.73 to 2.47; three studies, 360 participants; low-certainty evidence). The risk of seizure recurrence with six to 12 months of treatment compared with 24 months of treatment was inconclusive (OR 1.36, 95% CI 0.72 to 2.57; three studies, 385 participants; very low-certainty evidence). Two studies compared seizure recurrence with CT findings, and suggested that persistent and calcified lesions had a higher recurrence risk, and suggest longer duration of treatment with AEDs. One study reported no side effects, while the rest did not comment on side effects of the drugs. None of the studies addressed the quality of life of the participants. These studies had methodological deficiencies, such as small sample sizes, and a possibility of bias due to lack of blinding, which affect the results of the review.
Authors' conclusions: Despite neurocysticercosis being the most common cause of epilepsy worldwide, there is currently no evidence available regarding the use of AEDs as seizure prophylaxis among people presenting with symptoms other than seizures. For those presenting with seizures, there is no reliable evidence regarding the duration of treatment required. Therefore, there is a need for large scale randomised controlled trials to address these questions.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
DW: none known HC: none known CC: none known GKW: none known MS: none known TS: none known AM: none known BDM: none known
Figures






Update of
-
Antiepileptic drugs for seizure control in people with neurocysticercosis.Cochrane Database Syst Rev. 2019 Oct 14;10(10):CD009027. doi: 10.1002/14651858.CD009027.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 1;11:CD009027. doi: 10.1002/14651858.CD009027.pub4. PMID: 31608991 Free PMC article. Updated. Review.
References
References to studies included in this review
Gupta 2002 {published data only}
-
- Gupta M, Agarwal P, Khwaja GA, Chowdhury D, Sharma B, Bansal J, et al. Randomized prospective study of outcome of short term antiepileptic treatment in small single enhancing CT lesion in brain. Neurology India 2002;50(2):145-7. [PMID: ] - PubMed
Singhi 2003 {published data only}
Thussu 2002 {published data only}
-
- Thussu A, Arora A, Prabhakar S, Lal V, Sawhney IM. Acute symptomatic seizures due to single CT lesions: how long to treat with antiepileptic drugs? Neurology India 2002;50(2):141-4. [PMID: ] - PubMed
References to studies excluded from this review
Chang 1998 {published data only}
-
- Chang GY. Tiagabine HCl may be effective in complex partial epilepsy due to neurocysticercosis. Neurology 1998;50(4 Suppl 4):A200, Abstract no: P04.003.
Kaushal 2006 {published data only}
-
- Kaushal S, Rani A, Chopra SC, Singh G. Safety and efficacy of clobazam versus phenytoin sodium in the anti-epileptic drug treatment of solitary cysticercus granulomas. Neurology India 2006;54(2):157-60. [PMID: ] - PubMed
Lanchote 2002 {published data only}
-
- Lanchote VL, Garcia FS, Dreossi SA, Takayanagui OM. Pharmocokinetic interaction between albendazole sulfoxide enantiomers and antiepileptic drugs in patients with neurocysticercosis. Therapeutic Drug Monitoring 2002;24(3):338-45. [PMID: ] - PubMed
Santhosh 2021 {published data only (unpublished sought but not used)}
-
- Santhosh AP, Kumar Goyal M, Modi M, Kharbanda PS, Ahuja CK, Tandyala N, et al. Carbamazepine versus levetiracetam in epilepsy due to neurocysticercosis. Acta Neurologica Scandinavica 2021;143(3):242-7. [PMID: ] - PubMed
References to ongoing studies
CTRI/2019/01/017368 {published data only (unpublished sought but not used)}
-
- CTRI/2019/01/017368. A study comparing levetiracetam and valproic acid in preventing seizure in children diagnosed with neurocysticercosis. ctri.nic.in/Clinicaltrials/showallp.php?mid1=30301&EncHid=&userN... Registered 31 January 2019 (trial registered prospectively).
Additional references
Abba 2010
Daniels 2006
-
- Daniels TL, Moore TA. Neurocysticercosis in Kansas. Annals of Internal Medicine 2006;144(2):150-2. [PMID: ] - PubMed
DeGiorgio 2004
Del Brutto 1992
-
- Del Brutto OH, Santibañez R, Noboa CA, Aguirre R, Díaz E, Alarcón TA. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology 1992;42(2):389-92. [PMID: ] - PubMed
Del Brutto 1996
-
- Del Brutto OH, Campos X. Discontinuation of antiepileptic drugs in patients with calcified neurocysticercosis. Journal of Epilepsy 1996;9(4):231-3. [DOI: 10.1016/S0896-6974(96)00022-9] - DOI
Del Brutto 2001
Del Brutto 2005
-
- Del Brutto OH, Santibáñez R, Idrovo L, Rodrìguez S, Díaz-Calderón E, Navas C, et al. Epilepsy and neurocysticercosis in Atahualpa: a door to door survey in rural coastal Ecuador. Epilepsia 2005;46(4):583-7. [PMID: ] - PubMed
Dhawan 2011
-
- Dhawan VK. Pediatric neurocysticercosis. emedicine.medscape.com/article/999053-overview#a0199 (accessed 22 May 2014).
Ferreira 2002
-
- Ferreira LS, Li LM, Zanardi VA, Guerreiro MM. Number and viability of parasite influence seizure frequency in children with neurocysticercosis. Arquivos de Neuropsiquiatria 2002;60(4):909-11. [PMID: ] - PubMed
García 2002
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hauser 1992
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical supplement to chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Murthy 2007
-
- Murthy J, Rajshekhar G. Economic evaluation of seizures associated with solitary cysticercus granuloma. Neurology India 2007;55(1):42-5. [PMID: ] - PubMed
Pal 2000
Palacio 1998
-
- Palacio LG, Jiménez L Garcia HH, Jiménez ME, Sánchez JL, Noh J, et al. Neurocysticercosis in persons with epilepsy in Medellin, Colombia. Epilepsia 1998;39(12):1334-9. [PMID: ] - PubMed
Pradhan 2000
-
- Pradhan S, Kathuria MK, Gupta RK. Perilesional gliosis and seizure outcome:a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Annals of Neurology 2000;48(2):181-7. [PMID: ] - PubMed
Rajshekhar 2003
-
- Rajshekhar V. Solitary cerebral cysticercus granuloma. Epilepsia 2003;44(Suppl 1):25-8. [PMID: ] - PubMed
Rajshekhar 2004
-
- Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology 2004;62(12):2236-40. [PMID: ] - PubMed
Rajshekhar 2006
-
- Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore District, India. Neurology 2006;67(12):2135-9. [PMID: ] - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Sander 1996
Singhi 2000
-
- Singhi P, Ray M, Singhi S, Khandelwal N. Clinical spectrum of 500 children with neurocysticercosis and response to albendazole therapy. Journal of Child Neurology 2000;15(4):207-13. [PMID: ] - PubMed
Zafar 2013
-
- Zafar MJ. Neurocysticercosis. emedicine.medscape.com/article/1168656-overview#a0156 (accessed 22 May 2014).
References to other published versions of this review
Frackowiak 2019
Sharma 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

