HIV-1 Treatment Failure, Drug Resistance, and Clinical Outcomes in Perinatally Infected Children and Adolescents Failing First-Line Antiretroviral Therapy in Western Kenya
- PMID: 34723922
- PMCID: PMC8752470
- DOI: 10.1097/QAI.0000000000002850
HIV-1 Treatment Failure, Drug Resistance, and Clinical Outcomes in Perinatally Infected Children and Adolescents Failing First-Line Antiretroviral Therapy in Western Kenya
Abstract
Background: Long-term impact of drug resistance in perinatally infected children and adolescents living with HIV (CALWH) is poorly understood. We determined drug resistance and examined its long-term impact on failure and mortality in Kenyan CALWH failing first-line non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy (ART).
Setting: Academic Model Providing Access to Healthcare, western Kenya.
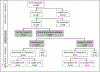
Methods: Participants were enrolled in 2010-2013 (timepoint 1) and a subsample re-enrolled after 4-7 years (timepoint 2). Viral load (VL) was performed on timepoint 1 samples, with genotyping of those with detectable VL. Primary endpoints were treatment failure (VL >1000 copies/mL) at and death before timepoint 2. Multinomial regression analysis was used to characterize resistance effect on death, failure, and loss-to-follow-up, adjusting for key variables.
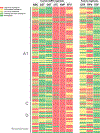
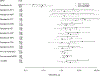
Results: The initial cohort (n = 480) was 52% (n = 251) female, median age 8 years, median CD4% 31%, 79% (n = 379) on zidovudine/abacavir + lamivudine + efavirenz/nevirapine for median 2 years. Of these, 31% (n = 149) failed at timepoint 1. Genotypes at timepoint 1, available on n = 128, demonstrated 93% (n = 119) extensive resistance, affecting second line. Of 128, 22 failed at timepoint 2, 17 died, and 32 were lost to follow-up before timepoint 2. Having >5 resistance mutations at timepoint 1 was associated with higher mortality [relative risk ratio (RRR) = 8.7, confidence interval (CI) 2.1 to 36.3] and loss to follow-up (RRR = 3.2, CI 1.1 to 9.2). Switching to second line was associated with lower mortality (RRR <0.05, CI <0.05 to 0.1) and loss to follow-up (RRR = 0.1, CI <0.05 to 0.3).
Conclusion: Extensive resistance and limited switch to second line in perinatally infected Kenyan CALWH failing first-line ART were associated with long-term failure and mortality. Findings emphasize urgency for interventions to sustain effective, life-long ART in this vulnerable population.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet 2019. [Available from: https://www.unaids.org/en/resources/fact-sheet.]
-
- UNAIDS. Understanding Fast-Track: Accelerating Action To End the AIDS Epidemic by 2030: Joint United Nations Programme on HIV/AIDS; 2014. [Available from: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Und....]
-
- Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with Scale-Up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries, January 2015-June 2016. MMWR Morbidity and mortality weekly report. 2016;65(47):1332–5. - PubMed
-
- Boerma RS, Boender TS, Bussink AP, Calis JCJ, Bertagnolio S, Rinke de Wit TF, et al. Suboptimal Viral Suppression Rates Among HIV-Infected Children in Low- and Middle-Income Countries: A Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(12):1645–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

