Neutrophil Extracellular Trap Density Increases With Increasing Histopathological Severity of Crohn's Disease
- PMID: 34724042
- PMCID: PMC9036391
- DOI: 10.1093/ibd/izab239
Neutrophil Extracellular Trap Density Increases With Increasing Histopathological Severity of Crohn's Disease
Abstract
Background: Intestinal neutrophil recruitment is a characteristic feature of the earliest stages of inflammatory bowel disease (IBD). Neutrophil elastase (NE) and myeloperoxidase (MPO) mediate the formation of neutrophil extracellular traps (NETs); NETs produce the bactericidal oxidant hypochlorous acid (HOCl), causing host tissue damage when unregulated. The project aim was to investigate the relationship between NET formation and clinical IBD in humans.
Methods: Human intestinal biopsies were collected from Crohn's disease (CD) patients, endoscopically categorized as unaffected, transitional, or diseased, and assigned a histopathological score.
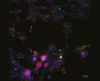
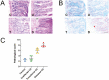
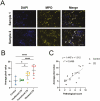
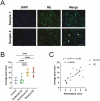
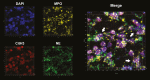
Results: A significant linear correlation was identified between pathological score and cell viability (TUNEL+). Immunohistochemical analysis revealed the presence of NET markers NE, MPO, and citrullinated histone (CitH3) that increased significantly with increasing histopathological score. Diseased specimens showed greater MPO+-immunostaining than control (P < .0001) and unaffected CD (P < .0001), with transitional CD specimens also showing greater staining than controls (P < .05) and unaffected CD (P < .05). Similarly, NE+-immunostaining was elevated significantly in diseased CD than controls (P < .0001) and unaffected CD (P < .0001) and was significantly higher in transitional CD than in controls (P < .0001) and unaffected CD (P < .0001). The CitH3+-immunostaining of diseased CD was significantly higher than controls (P < .05), unaffected CD (P < .0001) and transitional CD (P < .05), with transitional CD specimens showing greater staining than unaffected CD (P < .01). Multiplex immunohistochemistry with z-stacking revealed colocalization of NE, MPO, CitH3, and DAPI (cell nuclei), confirming the NET assignment.
Conclusion: These data indicate an association between increased NET formation and CD severity, potentially due to excessive MPO-mediated HOCl production in the extracellular domain, causing host tissue damage that exacerbates CD.
Keywords: inflammatory bowel disease; myeloperoxidase; neutrophil extracellular traps.
Plain language summary
Our data show for the first time that the density of neutrophil extracellular trap formed in the bowel of Crohn’s disease patients increases with increasing disease severity, suggesting that myeloperoxidase-mediated host-tissue damage may play a role in disease pathogenesis.
© 2021 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Comment in
-
Response to Low-density Granulocytes as Novel Biomarkers of Disease Activity in IBD.Inflamm Bowel Dis. 2023 Aug 1;29(8):e32. doi: 10.1093/ibd/izad133. Inflamm Bowel Dis. 2023. PMID: 37527412 Free PMC article. No abstract available.
-
Low-density Granulocytes as a Novel Biomarkers of Disease Activity in IBD.Inflamm Bowel Dis. 2023 Aug 1;29(8):e31. doi: 10.1093/ibd/izad136. Inflamm Bowel Dis. 2023. PMID: 37527413 No abstract available.
References
-
- Chami B, Martin NJJ, Dennis JM, Witting PK. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch Biochem Biophys. 2018;645:61–71. - PubMed
-
- Magro F, Langner C, Driessen A, et al. ; European Society of Pathology (ESP); European Crohn’s and Colitis Organisation (ECCO). . European consensus on the histopathology of inflammatory bowel disease. J Crohn’s Colitis. 2013;7:827–851. - PubMed
-
- Kim W-H. Atlas of Inflammatory Bowel Diseases. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015.
-
- Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

