Role of Akt isoforms in neuronal insulin signaling and resistance
- PMID: 34724097
- PMCID: PMC11073101
- DOI: 10.1007/s00018-021-03993-6
Role of Akt isoforms in neuronal insulin signaling and resistance
Abstract
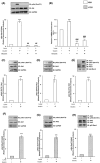
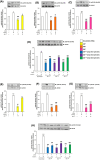
The aim of the present study was to determine the role of Akt isoforms in insulin signaling and resistance in neuronal cells. By silencing Akt isoforms individually and in pairs, in Neuro-2a and HT22 cells we observed that, in insulin-sensitive condition, Akt isoforms differentially reduced activation of AS160 and glucose uptake with Akt2 playing the major role. Under insulin-resistant condition, phosphorylation of all isoforms and glucose uptake were severely affected. Over-expression of individual isoforms in insulin-sensitive and resistant cells differentially reversed AS160 phosphorylation with concomitant reversal in glucose uptake indicating a compensatory role of Akt isoforms in controlling neuronal insulin signaling. Post-insulin stimulation Akt2 translocated to the membrane the most followed by Akt3 and Akt1, decreasing glucose uptake in the similar order in insulin-sensitive cells. None of the Akt isoforms translocated in insulin-resistant cells or high-fat-diet mediated diabetic mice brain cells. Based on our data, insulin-dependent differential translocation of Akt isoforms to the plasma membrane turns out to be the key factor in determining Akt isoform specificity. Thus, isoforms play parallel with predominant role by Akt2, and compensatory yet novel role by Akt1 and Akt3 to regulate neuronal insulin signaling, glucose uptake, and insulin-resistance.
Keywords: AS160; Akt1; Akt2; Akt3; Neuronal insulin resistance; Neuronal insulin signaling.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- Gupta A, Bisht B, Dey CS. Focal adhesion kinase negatively regulates neuronal insulin resistance. Biochim Biophys Acta. 2012;1822:1030–1037. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

