Phase 1 clinical trial evaluating safety, exposure and pharmacodynamics of BTK inhibitor tolebrutinib (PRN2246, SAR442168)
- PMID: 34724345
- PMCID: PMC8841436
- DOI: 10.1111/cts.13162
Phase 1 clinical trial evaluating safety, exposure and pharmacodynamics of BTK inhibitor tolebrutinib (PRN2246, SAR442168)
Abstract
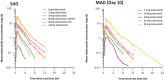
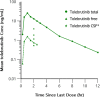
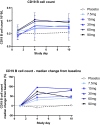
Bruton's tyrosine kinase (BTK), expressed in B cells and cells of innate immunity, including microglia, is an essential signaling element downstream of the B-cell receptor and Fc-receptors. Tolebrutinib (PRN2246, SAR442168) is a potent BTK inhibitor that covalently binds the kinase, resulting in durable inhibition with the potential to target inflammation in the periphery and central nervous system (CNS). Tolebrutinib crosses the blood-brain barrier and potently inhibits BTK in microglial cells isolated from the CNS. A first-in-human randomized, double-blind, placebo-controlled study of tolebrutinib was conducted. The trial design consisted of five single ascending dose arms with oral administration of a single dose of 5, 15, 30, 60, and 120 mg (n = 6 per arm, n = 2 placebo), five multiple ascending dose arms with oral administration of 7.5, 15, 30, 60, and 90 mg (n = 8 per arm, n = 2 placebo) over 10 days, and one arm (n = 4) in which cerebral spinal fluid (CSF) exposure was measured 2 h after a single 120 mg dose. Tolebrutinib was well-tolerated in the study and all treatment-related treatment emergent adverse events were mild. Tolebrutinib was rapidly absorbed following oral administration with a rapid half-life of ~ 2 h. Peripheral BTK occupancy was assessed at various timepoints by an enzyme-linked immunosorbent assay-based readout using an irreversible probe. Assessments demonstrated extensive and prolonged peripheral BTK occupancy at steady-state with once daily doses as low as 7.5 mg. Further, CSF exposure was demonstrated 2 h after administration at 120 mg.
© 2021 Sanofi. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
T.D.O., Y.X., J.S., D.E.K., M.R.F., C.L.L., P.A.N. and S.G.G. were, or are, employees of Principia Biopharma, a Sanofi Company. P.F.S. and S.H. were, or are employees of Certara, who received financial support from the Sponsor, Principia Biopharma. A.R. declared no competing interests for this work.
Figures

References
-
- Hauser SL, Bar‐Or A, Comi G, et al. Ocrelizumab versus interferon beta‐1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221‐234. - PubMed
-
- Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2016;376:209‐220. - PubMed
-
- Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung HP. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol. 2006;2:201‐211. - PubMed
-
- Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012;13:507‐514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

