2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party
- PMID: 34724563
- PMCID: PMC8718623
- DOI: 10.1182/blood.2021013626
2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party
Abstract
Measurable residual disease (MRD) is an important biomarker in acute myeloid leukemia (AML) that is used for prognostic, predictive, monitoring, and efficacy-response assessments. The European LeukemiaNet (ELN) MRD Working Party evaluated standardization and harmonization of MRD in an ongoing manner and has updated the 2018 ELN MRD recommendations based on significant developments in the field. New and revised recommendations were established during in-person and online meetings, and a 2-stage Delphi poll was conducted to optimize consensus. All recommendations are graded by levels of evidence and agreement. Major changes include technical specifications for next-generation sequencing-based MRD testing and integrative assessments of MRD irrespective of technology. Other topics include use of MRD as a prognostic and surrogate end point for drug testing; selection of the technique, material, and appropriate time points for MRD assessment; and clinical implications of MRD assessment. In addition to technical recommendations for flow- and molecular-MRD analysis, we provide MRD thresholds and define MRD response, and detail how MRD results should be reported and combined if several techniques are used. MRD assessment in AML is complex and clinically relevant, and standardized approaches to application, interpretation, technical conduct, and reporting are of critical importance.
© 2021 by The American Society of Hematology.
Figures
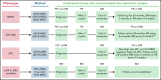
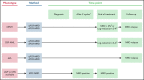
References
-
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008-1015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

