Bacterial Filamentation Drives Colony Chirality
- PMID: 34724813
- PMCID: PMC8561393
- DOI: 10.1128/mBio.01542-21
Bacterial Filamentation Drives Colony Chirality
Abstract
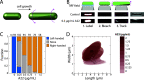
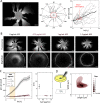
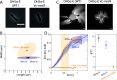
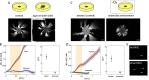
Chirality is ubiquitous in nature, with consequences at the cellular and tissue scales. As Escherichia coli colonies expand radially, an orthogonal component of growth creates a pinwheel-like pattern that can be revealed by fluorescent markers. To elucidate the mechanistic basis of this colony chirality, we investigated its link to left-handed, single-cell twisting during E. coli elongation. While chemical and genetic manipulation of cell width altered single-cell twisting handedness, colonies ceased to be chiral rather than switching handedness, and anaerobic growth altered colony chirality without affecting single-cell twisting. Chiral angle increased with increasing temperature even when growth rate decreased. Unifying these findings, we discovered that colony chirality was associated with the propensity for cell filamentation. Inhibition of cell division accentuated chirality under aerobic growth and generated chirality under anaerobic growth. Thus, regulation of cell division is intrinsically coupled to colony chirality, providing a mechanism for tuning macroscale spatial patterning. IMPORTANCE Chiral objects, such as amino acids, are distinguishable from their mirror image. For living systems, the fundamental mechanisms relating cellular handedness to chirality at the multicellular scale remain largely mysterious. Here, we use chemical, genetic, and environmental perturbations of Escherichia coli to investigate whether pinwheel patterns in bacterial colonies are directly linked to single-cell growth behaviors. We discover that chirality can be abolished without affecting single-cell twisting; instead, the degree of chirality was linked to the proportion of highly elongated cells at the colony edge. Inhibiting cell division boosted the degree of chirality during aerobic growth and even introduced chirality to otherwise achiral colonies during anaerobic growth. These findings reveal a fascinating connection between cell division and macroscopic colony patterning.
Keywords: A22; MreB; anaerobic growth; cell wall; cephalexin; chirality; colony growth; peptidoglycan; temperature; twisting.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
