mTOR Inhibitors Prevent CMV Infection through the Restoration of Functional αβ and γδ T cells in Kidney Transplantation
- PMID: 34725108
- PMCID: PMC8763189
- DOI: 10.1681/ASN.2020121753
mTOR Inhibitors Prevent CMV Infection through the Restoration of Functional αβ and γδ T cells in Kidney Transplantation
Abstract
Background: The reported association of mTOR-inhibitor (mTORi) treatment with a lower incidence of cytomegalovirus (CMV) infection in kidney transplant recipients (KTR) who are CMV seropositive (R+) remains unexplained.
Methods: The incidence of CMV infection and T-cell profile was compared between KTRs treated with mTORis and mycophenolic acid (MPA), and in vitro mTORi effects on T-cell phenotype and functions were analyzed.
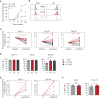
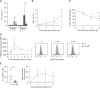
Results: In KTRs who were R+ and treated with MPA, both αβ and γδ T cells displayed a more dysfunctional phenotype (PD-1+, CD85j+) at day 0 of transplantation in the 16 KTRs with severe CMV infection, as compared with the 17 KTRs without or with spontaneously resolving CMV infection. In patients treated with mTORis (n=27), the proportion of PD-1+ and CD85j+ αβ and γδ T cells decreased, when compared with patients treated with MPA (n=44), as did the frequency and severity of CMV infections. mTORi treatment also led to higher proportions of late-differentiated and cytotoxic γδ T cells and IFNγ-producing and cytotoxic αβ T cells. In vitro, mTORis increased proliferation, viability, and CMV-induced IFNγ production of T cells and decreased PD-1 and CD85j expression in T cells, which shifted the T cells to a more efficient EOMESlow Hobithigh profile. In γδ T cells, the mTORi effect was related to increased TCR signaling.
Conclusion: Severe CMV replication is associated with a dysfunctional T-cell profile and mTORis improve T-cell fitness along with better control of CMV. A dysfunctional T-cell phenotype could serve as a new biomarker to predict post-transplantation infection and to stratify patients who should benefit from mTORi treatment.
Clinical trial registry name and registration number: Proportion of CMV Seropositive Kidney Transplant Recipients Who Will Develop a CMV Infection When Treated With an Immunosuppressive Regimen Including Everolimus and Reduced Dose of Cyclosporine Versus an Immunosuppressive Regimen With Mycophenolic Acid and Standard Dose of Cyclosporine A (EVERCMV), NCT02328963.
Keywords: CMV; CMV-specific immunity; kidney transplantation; mTOR inhibitors.
Copyright © 2022 by the American Society of Nephrology.
Figures






Comment in
-
Disarming the Old Foe. Restoring T-Cell Immune Function with mTor-Inhibitors to Tackle Cytomegalovirus Infection.J Am Soc Nephrol. 2022 Jan;33(1):6-8. doi: 10.1681/ASN.2021111471. J Am Soc Nephrol. 2022. PMID: 34969818 Free PMC article. No abstract available.
References
-
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. ; The Transplantation Society International CMV Consensus Group : The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 102: 900–931, 2018 - PubMed
-
- Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. : Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol 29: e2034, 2019 - PubMed
-
- Jarque M, Crespo E, Melilli E, Gutiérrez A, Moreso F, Guirado L, et al. : Cellular immunity to predict the risk of cytomegalovirus infection in kidney transplantation: A prospective, interventional, multicenter clinical trial. Clin Infect Dis 71: 2375–2385, 2020 - PubMed
-
- Kaminski H, Jarque M, Halfon M, Taton B, Di Ascia L, Pfirmann P, et al. : Different impact of rATG induction on CMV infection risk in D+R- and R+ KTRs. J Infect Dis 220: 761–771, 2019 - PubMed
-
- Appay V, van Lier RAW, Sallusto F, Roederer M: Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73: 975–983, 2008 - PubMed

