Dendritic cell paucity in mismatch repair-proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy
- PMID: 34725151
- PMCID: PMC8609309
- DOI: 10.1073/pnas.2105323118
Dendritic cell paucity in mismatch repair-proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy
Abstract
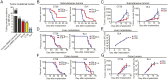
Liver metastasis is a major cause of mortality for patients with colorectal cancer (CRC). Mismatch repair-proficient (pMMR) CRCs make up about 95% of metastatic CRCs, and are unresponsive to immune checkpoint blockade (ICB) therapy. Here we show that mouse models of orthotopic pMMR CRC liver metastasis accurately recapitulate the inefficacy of ICB therapy in patients, whereas the same pMMR CRC tumors are sensitive to ICB therapy when grown subcutaneously. To reveal local, nonmalignant components that determine CRC sensitivity to treatment, we compared the microenvironments of pMMR CRC cells grown as liver metastases and subcutaneous tumors. We found a paucity of both activated T cells and dendritic cells in ICB-treated orthotopic liver metastases, when compared with their subcutaneous tumor counterparts. Furthermore, treatment with Feline McDonough sarcoma (FMS)-like tyrosine kinase 3 ligand (Flt3L) plus ICB therapy increased dendritic cell infiltration into pMMR CRC liver metastases and improved mouse survival. Lastly, we show that human CRC liver metastases and microsatellite stable (MSS) primary CRC have a similar paucity of T cells and dendritic cells. These studies indicate that orthotopic tumor models, but not subcutaneous models, should be used to guide human clinical trials. Our findings also posit dendritic cells as antitumor components that can increase the efficacy of immunotherapies against pMMR CRC.
Keywords: cancer immunotherapy; immune checkpoint blockade; mismatch repair–proficient colorectal cancer; orthotopic tumor model; tumor immune microenvironment.
Copyright © 2021 the Author(s). Published by PNAS.
Figures






References
-
- Larkin J., et al. ., Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019). - PubMed
-
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021). - PubMed
-
- Sung H., et al. ., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

