Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo
- PMID: 34725353
- PMCID: PMC8560862
- DOI: 10.1038/s41467-021-26518-y
Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo
Abstract
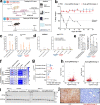
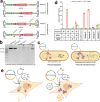
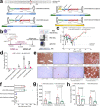
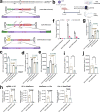
Adeno-associated virus (AAV) vectors are important delivery platforms for therapeutic genome editing but are severely constrained by cargo limits. Simultaneous delivery of multiple vectors can limit dose and efficacy and increase safety risks. Here, we describe single-vector, ~4.8-kb AAV platforms that express Nme2Cas9 and either two sgRNAs for segmental deletions, or a single sgRNA with a homology-directed repair (HDR) template. We also use anti-CRISPR proteins to enable production of vectors that self-inactivate via Nme2Cas9 cleavage. We further introduce a nanopore-based sequencing platform that is designed to profile rAAV genomes and serves as a quality control measure for vector homogeneity. We demonstrate that these platforms can effectively treat two disease models [type I hereditary tyrosinemia (HT-I) and mucopolysaccharidosis type I (MPS-I)] in mice by HDR-based correction of the disease allele. These results will enable the engineering of single-vector AAVs that can achieve diverse therapeutic genome editing outcomes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: A patent application has been filed on technologies related to this work by the University of Massachusetts Medical School [Names of inventors: Erik J. Sontheimer, Raed Ibraheim, Wen Xue, Aamir Mir, Alireza Edraki, Ildar Gainetdinov. Application number: 16/186,352. Status of application: Pending.] The following aspects of the manuscript are covered in the patent application: (1) Truncating of sgRNA sequence to create 121-nt sgRNA versions (Nme.sgRNA-121). (2) Truncating of sgRNA sequence to create 100-nt sgRNA versions (Nme.sgRNA-100). (3) Minimized all-in-one AAV.Nme2Cas9.sgRNA vector backbone to a packaging size of 4.4 Kb including its promoters and regulatory signals. (4) Dual-sgRNA AAV.NmeCas9 vector backbones. G.G. is a scientific co-founder of Voyager Therapeutics, Adrenas Therapeutics, and Aspa Therapeutics and holds equity in these companies. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Aspa Therapeutics, and other biopharmaceutical companies. E.J.S. is a co-founder and scientific advisor of Intellia Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

