Gait performance of adolescent mice assessed by the CatWalk XT depends on age, strain and sex and correlates with speed and body weight
- PMID: 34725364
- PMCID: PMC8560926
- DOI: 10.1038/s41598-021-00625-8
Gait performance of adolescent mice assessed by the CatWalk XT depends on age, strain and sex and correlates with speed and body weight
Abstract
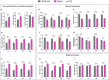
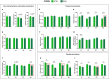
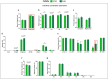
The automatization of behavioral tests assessing motor activity in rodent models is important for providing robust and reproducible results and evaluating new therapeutics. The CatWalk system is an observer-independent, automated and computerized technique for the assessment of gait performance in rodents. This method has previously been used in adult rodent models of CNS-based movement disorders such as Parkinson's and Huntington's diseases. As motor and gait abnormalities in neuropsychiatric disorders are observed during infancy and adolescence, it became important to validate the CatWalk XT in the gait analysis of adolescent mice and unravel factors that may cause variations in gait performance. Three adolescent wild-type inbred mouse strains, C57BL/6N, DBA/2 and FVB/N, were tested using the CatWalk XT (Version 10.6) for suitable detection settings to characterize several gait parameters at P32 and P42. The same detection settings being suitable for C57BL/6N and DBA/2 mice allowed a direct comparison between the two strains. On the other hand, due to their increased body weight and size, FVB/N mice required different detection settings. The CatWalk XT reliably measured the temporal, spatial, and interlimb coordination parameters in the investigated strains during adolescence. Additionally, significant effects of sex, development, speed and body weight within each strain confirmed the sensitivity of motor and gait functions to these factors. The CatWalk gait analysis of rodents during adolescence, taking the effect of age, strain, sex, speed and body weight into consideration, will decrease intra-laboratory discrepancies and increase the face validity of rodent models of neuropsychiatric disorders.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The CatWalk XT® is a valid tool for objective assessment of motor function in the acute phase after controlled cortical impact in mice.Behav Brain Res. 2020 Aug 17;392:112680. doi: 10.1016/j.bbr.2020.112680. Epub 2020 May 30. Behav Brain Res. 2020. PMID: 32479852
-
Focal lesion size poorly correlates with motor function after experimental traumatic brain injury in mice.PLoS One. 2022 Mar 16;17(3):e0265448. doi: 10.1371/journal.pone.0265448. eCollection 2022. PLoS One. 2022. PMID: 35294482 Free PMC article.
-
CatWalk XT gait parameters: a review of reported parameters in pre-clinical studies of multiple central nervous system and peripheral nervous system disease models.Front Behav Neurosci. 2023 Jun 7;17:1147784. doi: 10.3389/fnbeh.2023.1147784. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37351154 Free PMC article. Review.
-
Comprehensive characterization of motor and coordination functions in three adolescent wild-type mouse strains.Sci Rep. 2021 Mar 22;11(1):6497. doi: 10.1038/s41598-021-85858-3. Sci Rep. 2021. PMID: 33753800 Free PMC article.
-
Systematic data analysis and data mining in CatWalk gait analysis by heat mapping exemplified in rodent models for neurodegenerative diseases.J Neurosci Methods. 2019 Oct 1;326:108367. doi: 10.1016/j.jneumeth.2019.108367. Epub 2019 Jul 24. J Neurosci Methods. 2019. PMID: 31351096 Review.
Cited by
-
The mechanosensitive ion channel ASIC2 mediates both proprioceptive sensing and spinal alignment.Exp Physiol. 2024 Jan;109(1):135-147. doi: 10.1113/EP090776. Epub 2023 Mar 23. Exp Physiol. 2024. PMID: 36951012 Free PMC article.
-
Clicker Training Mice for Improved Compliance in the Catwalk Test.Animals (Basel). 2022 Dec 15;12(24):3545. doi: 10.3390/ani12243545. Animals (Basel). 2022. PMID: 36552465 Free PMC article.
-
Intermediate gray matter interneurons in the lumbar spinal cord play a critical and necessary role in coordinated locomotion.PLoS One. 2023 Oct 31;18(10):e0291740. doi: 10.1371/journal.pone.0291740. eCollection 2023. PLoS One. 2023. PMID: 37906544 Free PMC article.
-
Non-motor symptoms associated with progressive loss of dopaminergic neurons in a mouse model of Parkinson's disease.Front Neurosci. 2024 Apr 30;18:1375265. doi: 10.3389/fnins.2024.1375265. eCollection 2024. Front Neurosci. 2024. PMID: 38745938 Free PMC article.
-
CatWalk XT Gait Parameters Associated with Mouse Achilles Tendon Injury and Healing.Muscles Ligaments Tendons J. 2024 Apr-Jun;14(2):376-385. doi: 10.32098/mltj.02.2024.19. Muscles Ligaments Tendons J. 2024. PMID: 39619333 Free PMC article.
References
-
- Agyekum H. Motor symptoms of Parkinson’s disease—a review literature. Neurophysio Rehab. 2018 doi: 10.33805/2641-8991.112. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

