COVID-19 severity and mortality in patients with CLL: an update of the international ERIC and Campus CLL study
- PMID: 34725454
- PMCID: PMC8559135
- DOI: 10.1038/s41375-021-01450-8
COVID-19 severity and mortality in patients with CLL: an update of the international ERIC and Campus CLL study
Abstract
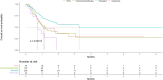
Patients with chronic lymphocytic leukemia (CLL) may be more susceptible to Coronavirus disease 2019 (COVID-19) due to age, disease, and treatment-related immunosuppression. We aimed to assess risk factors of outcome and elucidate the impact of CLL-directed treatments on the course of COVID-19. We conducted a retrospective, international study, collectively including 941 patients with CLL and confirmed COVID-19. Data from the beginning of the pandemic until March 16, 2021, were collected from 91 centers. The risk factors of case fatality rate (CFR), disease severity, and overall survival (OS) were investigated. OS analysis was restricted to patients with severe COVID-19 (definition: hospitalization with need of oxygen or admission into an intensive care unit). CFR in patients with severe COVID-19 was 38.4%. OS was inferior for patients in all treatment categories compared to untreated (p < 0.001). Untreated patients had a lower risk of death (HR = 0.54, 95% CI:0.41-0.72). The risk of death was higher for older patients and those suffering from cardiac failure (HR = 1.03, 95% CI:1.02-1.04; HR = 1.79, 95% CI:1.04-3.07, respectively). Age, CLL-directed treatment, and cardiac failure were significant risk factors of OS. Untreated patients had a better chance of survival than those on treatment or recently treated.
© 2021. The Author(s).
Conflict of interest statement
LS received advisory boards fees from AbbVie and Janssen; educational activity AstraZeneca. DAl received funding to attend symposia from Gilead and Bayer. MA received advisory boards fees from AbbVie, AstraZeneca, and Janssen-Cilag; travel support from AbbVie, and Novartis. DAn received honoraria from AbbVie, Janssen, and Roche. PB has received honoraria from Abbvie, Gilead and Janssen and research funding from Gilead. RC received speaker fees from Roche, Janssen, AbbVie, AstraZeneca, Celgene, BMS, Kite, and Takeda; received advisory board fees from Janssen, AbbVie, Celgene, BMS, Kite, Takeda, Incyte, Kyowa-Kirin, and ADCT; received travel and accommodation expenses from Roche, Janssen, AbbVie, Celgene, BMS, Kite, Takeda, and Pfizer; research grant from Pfizer. MD received honoraria for advisory board and research support from AstraZeneca, AbbVie, Roche, Gilead, and Janssen. BEi received consulting or advisory boards fees from Janssen, Roche, Novartis, AbbVie, Gilead, Celgene, ArQule, AstraZeneca, Oxford Biomedica (UK), and BeiGene; speaker/speaker’s bureau fees from Janssen, Gilead, Roche, AbbVie, Novartis, Celgene, Adaptive Biotechnologies, BioGene, and AstraZeneca; research support/research funding from Janssen, Gilead, Roche, AbbVie, BeiGene, and AstraZeneca; travel, accommodations, expenses from Janssen, Roche, Novartis, AbbVie, Gilead, and Celgene. MF received honoraria from Janssen and Gilead. JAG-M received honoraria for advisory board and speaker´s bureau from Mundipharma, Glaxo, AbbVie, Roche, Gilead, AstraZeneca, and Janssen; research support from Hoffman-La Roche, AbbVie, and Janssen. RG-S received educational grants from AbbVie, Janssen, and Novartis. EG received travel grants, honoraria as a consultant and/or speaker bureau for Janssen-Cilag, Roche, and AbbVie. MGDS received honoraria for consultancy/advisory boards with Roche, Janssen Cilag, Gilead, AbbVie, and BMS; research Grant from Gilead. YH received honoraria from AbbVie, Janssen, AstraZeneca, and Roche, outside the submitted work. JAH-R received honoraria and advisory boards fees from Janssen, AbbVie, AstraZeneca, Roche, Beigene, Gilead, and BMS-Celgene. OJ received honoraria from AbbVie, Janssen, and Roche. APK received honoraria from Janssen, BMS, AstraZeneca, Roche/Genentech; research money from Janssen, BMS, AstraZeneca, and Roche/Genentech. SK received Travel grant from Celgene; received research funding from Janssen and AbbVie. LL received honoraria from Roche, AbbVie, Janssen, and AstraZeneca. M-DL received travel expenses and advisory board compensation from Janssen, AbbVie, and Roche. AL-G received speaker’s bureau fees from Roche, Janssen, AbbVie, Celgene, Fresenius, Novonordisk; received advisory board participation fees from Janssen, and AbbVie; received travel and accommodation expenses from Roche, Janssen, and AbbVie. MMar received speaker bureau (invited speech) fees from Amgen; received honoraria as a consultant from Gilead. JM received honoraria and travel grants from AbbVie, Janssen, Roche, Gilead, and Takeda. MMat received research grant from GILEAD. FRM received research funding from Gilead; received advisory board participation fees from AbbVie, Gilead, Janssen, AstraZeneca, Takeda, and Roche; received speakers bureau fees from Gilead, Janssen, and AbbVie. TM received honoraria from AbbVie, Janssen, AstraZeneca, Gilead, Roche, and Alexion; received advisory board participation fees from AbbVie, AstraZeneca, Janssen, Alexion, Morphosys, and Sunesis. RM received honoraria from Janssen, and AbbVie. CUN received research funding and/or consultancy fees from AbbVie, AstraZeneca, Janssen, CSL Behring, and Takeda. MAP received advisory board participation fees from Janssen, AbbVie, AstraZeneca, and Merck; received speaker’s bureau fees from Janssen, AbbVie, AstraZeneca, Varifarma, and Merck. FMQ received advisory board participation fees from AstraZeneca and Janssen; received speakers bureau fees from Janssen. GR received consultancy fees and honoraria from AbbVie, AstraZeneca, and Janssen. GMR received honoraria from AbbVie, Gilead, and Janssen; received research funding from Gilead. MŠi received consultancy fees, advisory board participation fees, travel grants, and honoraria from Janssen, Gilead, Roche, AstraZeneca, and AbbVie. MŠp received honoraria from AbbVie, AstraZeneca, Gilead, Janssen, and Roche. PS received funding from Gilead; received advisory board participation fees from AbbVie and Janssen; received honorarium AbbVie, Janssen, and AstraZeneca. LT received advisory board participation fees from Janssen, Roche, AbbVie, Gilead, Takeda; research funding from Janssen, Roche, Takeda, and Gilead. EVDS participated in teaching activities for Amgen. MV received advisory board participation fees from Janssen, Roche, AstraZeneca; received travel expenses from Janssen and AbbVie. AV received speaker’s bureau fees from Italfarmaco and Gilead; received advisory board participation fees from Janssen and Takeda. CV received honoraria from Janssen. TW received research funding from Roche; received honoraria for advisory board, and research funding from Janssen; received honoraria, advisory board participation fees, and travel grant from AbbVie; received speaker’s bureau fees from Gilead. LYSS received advisory board participation fees from Gilead-Kite, Janssen, AbbVie, AstraZeneca, Beigene, Roche, Pfizer, Jazz, BMS, and Merck; received speaker’s bureau fees from Janssen, AbbVie, AstraZeneca, Gilead-Kite, Roche, Pfizer, and Merck. MC received honoraria from AbbVie, Gilead, Janssen, and AstraZeneca. AC received speaker’s bureau and advisory board fees from AbbVie, AstraZeneca, Gilead, and Janssen. RF received speaker’s bureau fees and/or advisory board participation fees from Amgen, Incyte, Janssen, Pfizer, AbbVie, Novartis, Servier, AstraZeneca, ACu received speaker’s bureau fees and advisory board participation fees from Abbvie, Asta-Zeneca, Gilead, Janssen KS received honoraria and research support from Janssen, Abbvie, AstraZeneca, Gilead. PG received honoraria from AbbVie, Arqule/MSD, AstraZeneca, Celgene/Juno/BMS, Janssen, Loxo/Lilly, Roche; Research support, AbbVie, AstraZeneca, Janssen, Gilead, Sunesis. TC, AK, GK, AAC, MB, DB, ACa, SC, J-GC, CC-G, LDP, MRDP, GDP, CD, MDi, DD, ME, SE-A, AE, BEs, LF, AF, HF, MFü, MG, AG, OG, YKH, TH, II, GI, AJ, OBK, EK, LKK, JL, DL, EL, LM, RM, IM, FM, MMor, MMot, RNR, JO, LO, MP, IP, VMP, GQ, KQ, RR, GS, AS, OS, NS, TT, DTR, SHT, MVG, RVK, EWS, MY, AR, EM have no conflict of interest to disclose.
Figures

Comment in
-
SARS-CoV-2 specific cellular response following COVID-19 vaccination in patients with chronic lymphocytic leukemia.Leukemia. 2022 Feb;36(2):562-565. doi: 10.1038/s41375-021-01500-1. Epub 2021 Dec 22. Leukemia. 2022. PMID: 34937858 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

