Dorsal striatal dopamine induces fronto-cortical hypoactivity and attenuates anxiety and compulsive behaviors in rats
- PMID: 34725486
- PMCID: PMC8559920
- DOI: 10.1038/s41386-021-01207-y
Dorsal striatal dopamine induces fronto-cortical hypoactivity and attenuates anxiety and compulsive behaviors in rats
Abstract
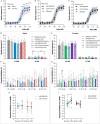
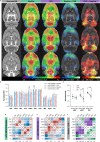
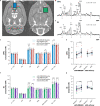
Dorsal striatal dopamine transmission engages the cortico-striato-thalamo-cortical (CSTC) circuit, which is implicated in many neuropsychiatric diseases, including obsessive-compulsive disorder (OCD). Yet it is unknown if dorsal striatal dopamine hyperactivity is the cause or consequence of changes elsewhere in the CSTC circuit. Classical pharmacological and neurotoxic manipulations of the CSTC and other brain circuits suffer from various drawbacks related to off-target effects and adaptive changes. Chemogenetics, on the other hand, enables a highly selective targeting of specific neuronal populations within a given circuit. In this study, we developed a chemogenetic method for selective activation of dopamine neurons in the substantia nigra, which innervates the dorsal striatum in the rat. We used this model to investigate effects of targeted dopamine activation on CSTC circuit function, especially in fronto-cortical regions. We found that chemogenetic activation of these neurons increased movement (as expected with increased dopamine release), rearings and time spent in center, while also lower self-grooming. Furthermore, this activation increased prepulse inhibition of the startle response in females. Remarkably, we observed reduced [18F]FDG metabolism in the frontal cortex, following dopamine activation in the dorsal striatum, while total glutamate levels- in this region were increased. This result is in accord with clinical studies of increased [18F]FDG metabolism and lower glutamate levels in similar regions of the brain of people with OCD. Taken together, the present chemogenetic model adds a mechanistic basis with behavioral and translational relevance to prior clinical neuroimaging studies showing deficits in fronto-cortical glucose metabolism across a variety of clinical populations (e.g. addiction, risky decision-making, compulsivity or obesity).
© 2021. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
MP is collaborating with the company Compass Pathways Plc (London, UK). MS is a member of the scientific advisory board of Roche Pharmaceuticals. The remaining authors have nothing to disclose.
Figures


References
-
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–11. 10.1016/s0960-9822(00)00593-5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

