Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells
- PMID: 34725504
- PMCID: PMC9499378
- DOI: 10.1038/s41551-021-00800-2
Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells
Abstract
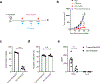
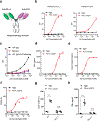
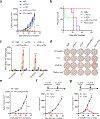
Bispecific T-cell engagers (BiTEs) preferentially targeting tumour-associated antigens and stimulating CD3-mediated signalling are being used in patients to treat acute B-cell lymphoblastic leukemia. However, the potency of BiTEs in solid tumours is limited by their short half-life and their severe toxicity at relevant therapeutic doses. Here we report the design and in vivo performance of a bispecific antibody that simultaneously targets the murine T-cell co-receptor CD3ε and the murine immune checkpoint programmed-death ligand 1 (PD-L1). In multiple syngeneic tumour models, the bispecific antibody generated higher antitumour immune responses than conventional BiTEs targeting tumour-associated antigens and CD3ε. We found that the durable antigen-specific T-cell responses resulted from the rejuvenation of CD8 T cells, owing to the blockade of PD-L1 on dendritic cells (but not on tumour cells) and co-stimulation by B7-1&2 (a peripheral membrane protein on dendritic cells). Bispecific T-cell engagers targeting dendritic cells rather than tumour cells may represent a general means of T-cell rejuvenation for durable cancer immunotherapy.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
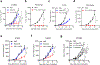







References
-
- Staerz UD, Kanagawa O & Bevan MJ Hybrid antibodies can target sites for attack by T cells. Nature 314, 628–631 (1985). - PubMed
-
- Garber K Bispecific antibodies rise again. Nature reviews. Drug discovery 13, 799–801 (2014). - PubMed
-
- Baeuerle PA & Reinhardt C Bispecific T-cell engaging antibodies for cancer therapy. Cancer research 69, 4941–4944 (2009). - PubMed
-
- Trabolsi A, Arumov A & Schatz JH T Cell-Activating Bispecific Antibodies in Cancer Therapy. Journal of immunology 203, 585–592 (2019). - PubMed
-
- Bargou R et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

