Monolithic, 3D-printed lab-on-disc platform for multiplexed molecular detection of SARS-CoV-2
- PMID: 34725537
- PMCID: PMC8550893
- DOI: 10.1016/j.snb.2021.130998
Monolithic, 3D-printed lab-on-disc platform for multiplexed molecular detection of SARS-CoV-2
Abstract
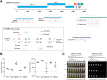
Multiplexed detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rather than detection targeting a single gene is crucial to ensure more accurate coronavirus disease 2019 (COVID-19) diagnostics. Here, we develop a monolithic, 3D-printed, lab-on-disc platform for multiplexed molecular detection of SARS-CoV-2. The centrifugal lab-on-disc is fabricated in one step using simple 3D printing technology, circumventing the need for aligning and binding multiple layers. By combining isothermal amplification technology, this lab-on-disc platform is capable of simultaneously detecting the nucleoprotein and envelope genes of SARS-CoV-2 as well as an internal control of the human POP7 gene. Within a 50-minute incubation period, 100 copies SARS-CoV-2 RNA can be detected through visual observation according to color and fluorescence changes in the disc. Further, we clinically validated the lab-on-disc platform by testing 20 nasopharyngeal swab samples and demonstrated a sensitivity of 100% and an accuracy of 95%. Therefore, the monolithic, 3D-printed, lab-on-disc platform provides simple, rapid, disposable, sensitive, reliable, and multiplexed molecular detection of SARS-CoV-2, holding promise for COVID-19 diagnostics at the point of care.
Keywords: 3D printing; COVID-19; Lab-on-disc; Multiplexed molecular detection; SARS-CoV-2.
© 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
We confirm that we have no known competing financial interests or personal relationships.
Figures





Similar articles
-
Autonomous lab-on-paper for multiplexed, CRISPR-based diagnostics of SARS-CoV-2.Lab Chip. 2021 Jul 13;21(14):2730-2737. doi: 10.1039/d1lc00293g. Lab Chip. 2021. PMID: 34100058 Free PMC article.
-
Lab-on-a-Disc for Point-of-Care Infection Diagnostics.Acc Chem Res. 2021 Oct 5;54(19):3643-3655. doi: 10.1021/acs.accounts.1c00367. Epub 2021 Sep 13. Acc Chem Res. 2021. PMID: 34516092
-
A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma.Nat Biomed Eng. 2022 Aug;6(8):968-978. doi: 10.1038/s41551-022-00919-w. Epub 2022 Aug 8. Nat Biomed Eng. 2022. PMID: 35941191 Free PMC article.
-
CLEVER assay: A visual and rapid RNA extraction-free detection of SARS-CoV-2 based on CRISPR-Cas integrated RT-LAMP technology.J Appl Microbiol. 2022 Aug;133(2):410-421. doi: 10.1111/jam.15571. Epub 2022 Apr 18. J Appl Microbiol. 2022. PMID: 35396760 Free PMC article.
-
Isothermal amplification-based assays for rapid and sensitive detection of severe acute respiratory syndrome coronavirus 2: Opportunities and recent developments.Rev Med Virol. 2022 Mar;32(2):e2274. doi: 10.1002/rmv.2274. Epub 2021 Jul 3. Rev Med Virol. 2022. PMID: 34216498 Free PMC article. Review.
Cited by
-
Microfluidics for COVID-19: From Current Work to Future Perspective.Biosensors (Basel). 2023 Jan 20;13(2):163. doi: 10.3390/bios13020163. Biosensors (Basel). 2023. PMID: 36831930 Free PMC article. Review.
-
Accuracy of clustered regularly interspaced short palindromic repeats (CRISPR) to diagnose COVID-19, a meta-analysis.Microb Pathog. 2022 Apr;165:105498. doi: 10.1016/j.micpath.2022.105498. Epub 2022 Mar 25. Microb Pathog. 2022. PMID: 35341958 Free PMC article.
-
Enhancing Mixing Performance in a Rotating Disk Mixing Chamber: A Quantitative Investigation of the Effect of Euler and Coriolis Forces.Micromachines (Basel). 2022 Jul 29;13(8):1218. doi: 10.3390/mi13081218. Micromachines (Basel). 2022. PMID: 36014138 Free PMC article.
-
Emerging trends of biomedical nanotechnology in nutrition, health monitoring and disease diagnosis.3 Biotech. 2025 Jun;15(6):152. doi: 10.1007/s13205-025-04291-9. Epub 2025 May 5. 3 Biotech. 2025. PMID: 40336812 Review.
-
Atomically dispersed Mn boosting photoelectrochemical SARS-CoV-2 spike protein immunosensing on carbon nitride.J Environ Chem Eng. 2022 Dec;10(6):108697. doi: 10.1016/j.jece.2022.108697. Epub 2022 Oct 3. J Environ Chem Eng. 2022. PMID: 36213529 Free PMC article.
References
-
- WHO, WHO Coronavirus (COVID-19) Dashboard, https://covid19whoint/, (2021).
-
- Ben-Ami R., Klochendler A., Seidel M., Sido T., Gurel-Gurevich O., Yassour M., Meshorer E., Benedek G., Fogel I., Oiknine-Djian E., Gertler A., Rotstein Z., Lavi B., Dor Y., Wolf D.G., Salton M., Drier Y., Hebrew T. University-Hadassah COVID- Diagnosis, large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin. Microbiol. Infect. 2020;26:1248–1253. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

