Effect of HIV-1 Infection on Angiopoietin 1 and 2 Levels and Measures of Microvascular and Macrovascular Endothelial Dysfunction
- PMID: 34726064
- PMCID: PMC8751943
- DOI: 10.1161/JAHA.121.021397
Effect of HIV-1 Infection on Angiopoietin 1 and 2 Levels and Measures of Microvascular and Macrovascular Endothelial Dysfunction
Abstract
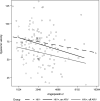
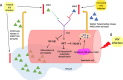
Background Individuals infected with HIV have an increased risk of developing cardiovascular disease; yet, the underlying mechanisms remain unknown. Recent evidence has implicated the Tie-2 tyrosine kinase receptor system and its associated ligands ANG1 (angiopoietin 1) and ANG2 (angiopoietin 2) in maintaining vascular homeostasis. In the general population, lower ANG1 levels and higher ANG2 levels are strongly correlated with the development of cardiovascular disease. In this study, we aim to investigate the associations of HIV infection with angiopoietin levels and endothelial dysfunction. Methods and Results In this cross-sectional study, we compared measures of ANG1, ANG2, and endothelial dysfunction using flow-mediated vasodilation of the brachial artery in 39 untreated subjects infected with HIV, 47 treated subjects infected with HIV, and 46 uninfected subjects from the SCOPE (Observational Study of the Consequences of the Protease Inhibitor Era) cohort. Compared with uninfected controls, treated individuals infected with HIV had 53.1% lower mean ANG1 levels (P<0.01) and similar ANG2 levels. On the other hand, untreated individuals infected with HIV had similar ANG1 levels, and 29.2% had higher ANG2 levels (P<0.01) compared with uninfected controls. When compared with individuals with untreated HIV infection, those with treated HIV infection had 56% lower ANG1 levels (P<0.01) and 22% lower ANG2 levels (P<0.01).Both treated and untreated HIV infection were associated with significant impairment in hyperemic velocity, a key measure of microvascular dysfunction (median 61 versus 72 cm/s, P<0.01), compared with uninfected controls (median 73 cm/s). This difference persisted after adjustment for ANG1 and ANG2 levels. Interestingly, when compared with untreated individuals infected with HIV, treated individuals infected with HIV had worse hyperemic velocity (-12.35 cm/s, P=0.05). In contrast, HIV status, ANG1 levels, and ANG2 levels were not associated with macrovascular dysfunction as measured by flow-mediated dilatation and brachial artery diameter, 2 other measures of vascular homeostasis. Conclusions HIV infection affects the balance between levels of ANG1 and ANG2 and may disturb endothelial homeostasis through disruption of vascular homeostasis. Individuals with treated HIV had decreased ANG1 levels and similar ANG2 levels, whereas individuals with untreated HIV had similar ANG1 levels and increased ANG2 levels, suggesting that treatment status may alter the balance between ANG1 and ANG2. HIV also promotes endothelial dysfunction via impairment of microvascular dysfunction, independent of the Tie-2 receptor system; the finding of worse microvascular dysfunction in the setting of treated HIV infection may reflect the impact of viral persistence on the microvasculature or toxicities of specific antiretroviral regimens. Further research to clarify the mechanism of HIV-mediated endothelial dysfunction is necessary to advance treatment of cardiovascular complications of HIV infection.
Keywords: HIV; angiopoietin 1; angiopoietin 2; endothelial dysfunction; endothelial homeostasis.
Figures
Similar articles
-
Endothelial permeability following coronary artery bypass grafting: an observational study on the possible role of angiopoietin imbalance.Crit Care. 2016 Mar 6;20:51. doi: 10.1186/s13054-016-1238-0. Crit Care. 2016. PMID: 26951111 Free PMC article.
-
Fetoplacental regional variations in the expression of angiopoietin-1, angiopoietin-2, and Tie2 in normal-term and near-term pregnancies.J Matern Fetal Neonatal Med. 2016 Nov;29(21):3421-8. doi: 10.3109/14767058.2015.1136282. Epub 2016 Mar 3. J Matern Fetal Neonatal Med. 2016. PMID: 26752164
-
Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface.Cell Signal. 2010 Mar;22(3):527-32. doi: 10.1016/j.cellsig.2009.11.007. Cell Signal. 2010. PMID: 19922791 Free PMC article.
-
Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes.J Endocrinol Invest. 2016 Nov;39(11):1235-1246. doi: 10.1007/s40618-016-0502-0. Epub 2016 Jun 25. J Endocrinol Invest. 2016. PMID: 27344309 Review.
-
Angiopoietin 2 signal complexity in cardiovascular disease and cancer.Life Sci. 2019 Dec 15;239:117080. doi: 10.1016/j.lfs.2019.117080. Epub 2019 Nov 19. Life Sci. 2019. PMID: 31756341 Review.
Cited by
-
The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway.Transl Neurosci. 2023 Aug 23;14(1):20220302. doi: 10.1515/tnsci-2022-0302. eCollection 2023 Jan 1. Transl Neurosci. 2023. PMID: 37635842 Free PMC article.
-
Vascular endothelial growth factor A: friend or foe in the pathogenesis of HIV and SARS-CoV-2 infections?Front Cell Infect Microbiol. 2025 Feb 11;14:1458195. doi: 10.3389/fcimb.2024.1458195. eCollection 2024. Front Cell Infect Microbiol. 2025. PMID: 40008234 Free PMC article. Review.
-
Cardiac and Renal Comorbidities in Aging People Living With HIV.Circ Res. 2024 May 24;134(11):1636-1660. doi: 10.1161/CIRCRESAHA.124.323948. Epub 2024 May 23. Circ Res. 2024. PMID: 38781295 Free PMC article. Review.
-
The management outcomes of chronic subdural hematoma in HIV-Infected individuals.BMC Neurol. 2025 Aug 20;25(1):341. doi: 10.1186/s12883-025-04364-5. BMC Neurol. 2025. PMID: 40830446 Free PMC article.
References
-
- Freiberg MS, Chang C‐C, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So‐Armah KA, Vasan RS, Oursler KA, Gottdiener J, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. doi: 10.1001/jamacardio.2017.0264 - DOI - PMC - PubMed
-
- Hsu JC, Li Y, Marcus GM, Hsue PY, Scherzer R, Grunfeld C, Shlipak MG. Atrial fibrillation and atrial flutter in human immunodeficiency virus‐infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol. 2013;61:2288–2295. doi: 10.1016/j.jacc.2013.03.022 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

