Luminescent PLGA Nanoparticles for Delivery of Darunavir to the Brain and Inhibition of Matrix Metalloproteinase-9, a Relevant Therapeutic Target of HIV-Associated Neurological Disorders
- PMID: 34726377
- PMCID: PMC9297288
- DOI: 10.1021/acschemneuro.1c00436
Luminescent PLGA Nanoparticles for Delivery of Darunavir to the Brain and Inhibition of Matrix Metalloproteinase-9, a Relevant Therapeutic Target of HIV-Associated Neurological Disorders
Abstract
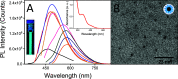
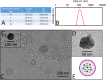
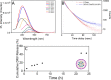
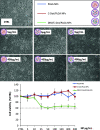
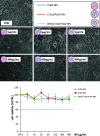
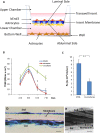
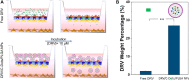
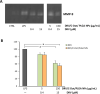
Human immunodeficiency virus (HIV) can independently replicate in the central nervous system (CNS) causing neurocognitive impairment even in subjects with suppressed plasma viral load. The antiretroviral drug darunavir (DRV) has been approved for therapy of HIV-infected patients, but its efficacy in the treatment of HIV-associated neurological disorders (HAND) is limited due to the low penetration through the blood-brain barrier (BBB). Therefore, innovations in DRV formulations, based on its encapsulation in optically traceable nanoparticles (NPs), may improve its transport through the BBB, providing, at the same time, optical monitoring of drug delivery within the CNS. The aim of this study was to synthesize biodegradable polymeric NPs loaded with DRV and luminescent, nontoxic carbon dots (C-Dots) and investigate their ability to permeate through an artificial BBB and to inhibit in vitro matrix metalloproteinase-9 (MMP-9) that represents a factor responsible for the development of HIV-related neurological disorders. Biodegradable poly(lactic-co-glycolic) acid (PLGA)-based nanoformulations resulted characterized by an average hydrodynamic size less than 150 nm, relevant colloidal stability in aqueous medium, satisfactory drug encapsulation efficiency, and retained emitting optical properties in the visible region of the electromagnetic spectrum. The assay on the BBB artificial model showed that a larger amount of DRV was able to cross BBB when incorporated in the PLGA NPs and to exert an enhanced inhibition of matrix metalloproteinase-9 (MMP-9) expression levels with respect to free DRV. The overall results reveal the great potential of this class of nanovectors of DRV for an efficacious treatment of HANDs.
Keywords: HANDs; MMP-9; PLGA nanoparticles; blood−brain barrier; carbon dots; darunavir.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Darunavir Nanoformulation Suppresses HIV Pathogenesis in Macrophages and Improves Drug Delivery to the Brain in Mice.Pharmaceutics. 2024 Apr 19;16(4):555. doi: 10.3390/pharmaceutics16040555. Pharmaceutics. 2024. PMID: 38675216 Free PMC article.
-
An Elvitegravir Nanoformulation Crosses the Blood-Brain Barrier and Suppresses HIV-1 Replication in Microglia.Viruses. 2020 May 20;12(5):564. doi: 10.3390/v12050564. Viruses. 2020. PMID: 32443728 Free PMC article.
-
Nanoparticle-in-microparticle oral drug delivery system of a clinically relevant darunavir/ritonavir antiretroviral combination.Acta Biomater. 2018 Jul 1;74:344-359. doi: 10.1016/j.actbio.2018.04.045. Epub 2018 May 1. Acta Biomater. 2018. PMID: 29723705
-
PLGA Nanoparticle-Based Formulations to Cross the Blood-Brain Barrier for Drug Delivery: From R&D to cGMP.Pharmaceutics. 2021 Apr 6;13(4):500. doi: 10.3390/pharmaceutics13040500. Pharmaceutics. 2021. PMID: 33917577 Free PMC article. Review.
-
Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier.Curr Med Chem. 2013;20(17):2212-25. doi: 10.2174/0929867311320170006. Curr Med Chem. 2013. PMID: 23458620 Review.
Cited by
-
Neuroinflammation, Blood-Brain Barrier, and HIV Reservoirs in the CNS: An In-Depth Exploration of Latency Mechanisms and Emerging Therapeutic Strategies.Viruses. 2025 Apr 16;17(4):572. doi: 10.3390/v17040572. Viruses. 2025. PMID: 40285014 Free PMC article. Review.
-
Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD.Nutrients. 2022 Mar 8;14(6):1133. doi: 10.3390/nu14061133. Nutrients. 2022. PMID: 35334792 Free PMC article.
-
PLGA-Encapsulated Elvitegravir and Curcumin Modulates ART Penetration, Oxidative Stress, and Inflammation.Brain Sci. 2025 Mar 21;15(4):328. doi: 10.3390/brainsci15040328. Brain Sci. 2025. PMID: 40309788 Free PMC article.
-
The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke.Pharmaceutics. 2023 Sep 14;15(9):2322. doi: 10.3390/pharmaceutics15092322. Pharmaceutics. 2023. PMID: 37765291 Free PMC article. Review.
-
Darunavir Nanoformulation Suppresses HIV Pathogenesis in Macrophages and Improves Drug Delivery to the Brain in Mice.Pharmaceutics. 2024 Apr 19;16(4):555. doi: 10.3390/pharmaceutics16040555. Pharmaceutics. 2024. PMID: 38675216 Free PMC article.
References
-
- Antinori A.; Arendt G.; Becker J. T.; Brew B. J.; Byrd D. A.; Cherner M.; Clifford D. B.; Cinque P.; Epstein L. G.; Goodkin K.; Gisslen M.; Grant I.; Heaton R. K.; Joseph J.; Marder K.; Marra C. M.; McArthur J. C.; Nunn M.; Price R. W.; Pulliam L.; Robertson K. R.; Sacktor N.; Valcour V.; Wojna V. E. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. 10.1212/01.WNL.0000287431.88658.8b. - DOI - PMC - PubMed
-
- King J. M. J. B.; Gannon P. J.; Akaj C.. Persistence of HIV-Associated Neurocognitive Disorders in the Era of Antiretroviral Therapy. In Therapy, Current Perspectives in HIV Infection; Saxena S. K., Eds.; IntechOpen, 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

