Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant
- PMID: 34726481
- PMCID: PMC10763627
- DOI: 10.1126/science.abl9551
Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant
Abstract
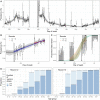
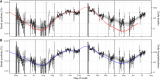
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections were rising during early summer 2021 in many countries as a result of the Delta variant. We assessed reverse transcription polymerase chain reaction swab positivity in the Real-time Assessment of Community Transmission–1 (REACT-1) study in England. During June and July 2021, we observed sustained exponential growth with an average doubling time of 25 days, driven by complete replacement of the Alpha variant by Delta and by high prevalence at younger, less-vaccinated ages. Prevalence among unvaccinated people [1.21% (95% credible interval 1.03%, 1.41%)] was three times that among double-vaccinated people [0.40% (95% credible interval 0.34%, 0.48%)]. However, after adjusting for age and other variables, vaccine effectiveness for double-vaccinated people was estimated at between ~50% and ~60% during this period in England. Increased social mixing in the presence of Delta had the potential to generate sustained growth in infections, even at high levels of vaccination.
Figures


References
-
- Folegatti P. M., Ewer K. J., Aley P. K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E. A., Dold C., Faust S. N., Finn A., Flaxman A. L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A. M., Pollock K. M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A. D., Hill A. V. S., Lambe T., Gilbert S. C., Pollard A. J., Oxford COVID Vaccine Trial Group , Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478 (2020). 10.1016/S0140-6736(20)31604-4 - DOI - PMC - PubMed
-
- Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W. Jr., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C., Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). 10.1056/NEJMoa2034577 - DOI - PMC - PubMed
-
- Johns Hopkins University Coronavirus Resource Center , https://coronavirus.jhu.edu/.
-
- M. S. Dhar, R. Marwal, V. S. Radhakrishnan, K. Ponnusamy, B. Jolly, R. C. Bhoyar, V. Sardana, S. Naushin, M. Rophina, T. A. Mellan, S. Mishra, C. Whittaker, S. Fatihi, M. Datta, P. Singh, U. Sharma, R. Ujjainiya, N. Batheja, M. K. Divakar, M. K. Singh, M. Imran, V. Senthivel, R. Maurya, N. Jha, P. Mehta, A. Vivekanand, P. Sharma, V. R. Arvinden, U. Chaudhary, N. Soni, L. Thukral, S. Flaxman, S. Bhatt, R. Pandey, D. Dash, M. Faruq, H. Lall, H. Gogia, P. Madan, S. Kulkarni, H. Chauhan, S. Sengupta, S. Kabra, R. K. Gupta, S. K. Singh, A. Agrawal, P. Rakshit, The Indian SARS-CoV-2 Genomics Consortium (INSACOG), Genomic characterization and Epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. medRxiv 21258076 [preprint] (2021). 10.1101/2021.06.02.21258076 - DOI
-
- Coronavirus (COVID-19) Vaccinations. Our World In Data; https://ourworldindata.org/covid-vaccinations.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

