The Role of Real-World Evidence in FDA-Approved New Drug and Biologics License Applications
- PMID: 34726771
- PMCID: PMC9299054
- DOI: 10.1002/cpt.2474
The Role of Real-World Evidence in FDA-Approved New Drug and Biologics License Applications
Abstract
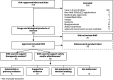
The US Food and Drug Administration (FDA) is open to accepting real-world evidence (RWE) to support its assessment of medical products. However, RWE stakeholders lack a shared understanding of FDA's evidentiary expectations for the use of RWE in applications for new drugs and biologics. We conducted a systematic review of publicly available FDA approval documents from January 2019 to June 2021. We sought to quantify, by year, how many approvals incorporated RWE in any form, and the intended use of RWE in those applications. Among approvals with RWE intended to support safety and/or effectiveness, we classified whether and how those studies impacted FDA's benefit-risk considerations, whether those studies were incorporated into the product label, and the therapeutic area of the medical product. Finally, we qualified FDA's documented feedback where available. We found that 116 approvals incorporated RWE in any form, with the proportion of approvals incorporating RWE increasing each year. Of these approvals, 88 included an RWE study intended to provide evidence of safety or effectiveness. Among these 88 approvals, 65 of the studies influenced FDA's final decision and 38 were included in product labels. The 88 approvals spanned 18 therapeutic areas. FDA's feedback on RWE study quality included methodological issues, sample size concerns, omission of patient level data, and other limitations. Based on these findings, we would anticipate that future guidance on FDA's evidentiary expectations of RWE use will incorporate fit-for-purpose real-world data selection and careful attention to study design and analysis.
© 2021 Aetion, Inc. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employees of and hold stock options or equity in Aetion, Inc. No other interests are declared.
Figures
References
-
- FDA . Framework for FDA’s Real‐World Evidence Program. Published December 2018 <https://www.fda.gov/media/120060/download>. Accessed July 26, 2021.
-
- EMA regulatory science to 2025. Published online 2020 <https://www.ema.europa.eu/en/documents/regulatory‐procedural‐guideline/e...>. Accessed August 9, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources