Proteome-Wide Profiling of Cellular Targets Modified by Dopamine Metabolites Using a Bio-Orthogonally Functionalized Catecholamine
- PMID: 34726906
- PMCID: PMC9872492
- DOI: 10.1021/acschembio.1c00629
Proteome-Wide Profiling of Cellular Targets Modified by Dopamine Metabolites Using a Bio-Orthogonally Functionalized Catecholamine
Abstract
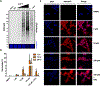
Selective death of midbrain dopaminergic neurons is a hallmark pathology of Parkinson's disease (PD), but the molecular mechanisms that initiate the cascade of events resulting in neurodegeneration in PD remain unclear. Compelling evidence suggests that dysregulation of dopamine (DA) induces neuronal stress and damage responses that are operative processes in striatal degeneration preceding PD-like symptoms. Improper DA sequestration to vesicles raises cytosolic DA levels, which is rapidly converted into electrophilic dopaquinone species (DQs) that react readily with protein nucleophiles forming covalent modifications that alter the native structure and function of proteins. These so-called DA-protein adducts (DPAs) have been reported to play a role in neurotoxicity, and their abundance with respect to neurodegeneration has been linked to clinical and pathological features of PD that suggest that they play a causal role in PD pathogenesis. Therefore, characterizing DPAs is a critical first step in understanding the susceptibility of midbrain dopaminergic neurons during PD. To help achieve this goal, we report here a novel DA-mimetic (DAyne) containing a biorthogonal alkyne handle that exhibits a reactivity profile similar to DA in aqueous buffers. By linking DPAs formed with DAyne to a fluorescent reporter molecule, DPAs were visualized in fixed cells and within lysates. DAyne enabled global mapping of cellular proteins affected by DQ modification and their bioactive pathways through enrichment. Our proteomic profiling of DPAs in neuronal SH-SY5Y cells indicates that proteins susceptible to DPA formation are extant throughout the proteome, potentially influencing several diverse biological pathways involved in PD such as endoplasmic reticulum (ER) stress, cytoskeletal instability, proteotoxicity, and clathrin function. We validated that a protein involved in the ER stress pathway, protein disulfide isomerase 3 (PDIA3), which was enriched in our chemoproteomic analysis, is functionally inhibited by DA, providing evidence that dysregulated cellular DA may induce or exacerbate ER stress. Thus, DAyne provided new mechanistic insights into DA toxicity that may be observed during PD by enabling characterization of DPAs generated reproducibly at physiologically relevant quinone exposures. We anticipate our design and application of this reactivity-based probe will be generally applicable for clarifying mechanisms of metabolic quinone toxicity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
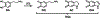





References
-
- Poewe W; Seppi K; Tanner CM; Halliday GM; Brundin P; Volkmann J; Schrag A-E; Lang AE Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. - PubMed
-
- Zecca L; Fariello R; Riederer P; Sulzer D; Gatti A; Tampellini D The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002, 510, 216–220. - PubMed
-
- Monzani E; Nicolis S; Dell’Acqua S; Capucciati A; Bacchella C; Zucca FA; Mosharov EV; Sulzer D; Zecca L; Casella L Dopamine, Oxidative Stress and Protein-Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem., Int. Ed. Engl 2019, 58, 6512–6527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

