Hypoxia induces DOT1L in articular cartilage to protect against osteoarthritis
- PMID: 34727094
- PMCID: PMC8783684
- DOI: 10.1172/jci.insight.150451
Hypoxia induces DOT1L in articular cartilage to protect against osteoarthritis
Abstract
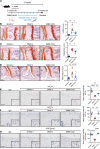
Osteoarthritis is the most prevalent joint disease worldwide, and it is a leading source of pain and disability. To date, this disease lacks curative treatment, as underlying molecular mechanisms remain largely unknown. The histone methyltransferase DOT1L protects against osteoarthritis, and DOT1L-mediated H3K79 methylation is reduced in human and mouse osteoarthritic joints. Thus, restoring DOT1L function seems to be critical to preserve joint health. However, DOT1L-regulating molecules and networks remain elusive, in the joint and beyond. Here, we identified transcription factors and networks that regulate DOT1L gene expression using a potentially novel bioinformatics pipeline. Thereby, we unraveled a possibly undiscovered link between the hypoxia pathway and DOT1L. We provide evidence that hypoxia enhanced DOT1L expression and H3K79 methylation via hypoxia-inducible factor-1 α (HIF1A). Importantly, we demonstrate that DOT1L contributed to the protective effects of hypoxia in articular cartilage and osteoarthritis. Intra-articular treatment with a selective hypoxia mimetic in mice after surgical induction of osteoarthritis restored DOT1L function and stalled disease progression. Collectively, our data unravel a molecular mechanism that protects against osteoarthritis with hypoxia inducing DOT1L transcription in cartilage. Local treatment with a selective hypoxia mimetic in the joint restores DOT1L function and could be an attractive therapeutic strategy for osteoarthritis.
Keywords: Aging; Bone Biology; Cartilage; Osteoarthritis.
Figures





Comment in
-
Articular cartilage hypoxia is a potential target for OA therapy.Nat Rev Rheumatol. 2022 Jan;18(1):3. doi: 10.1038/s41584-021-00729-5. Nat Rev Rheumatol. 2022. PMID: 34848884 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

