The seroprevalence of SARS-CoV-2 during the first wave in Europe 2020: A systematic review
- PMID: 34727115
- PMCID: PMC8562786
- DOI: 10.1371/journal.pone.0250541
The seroprevalence of SARS-CoV-2 during the first wave in Europe 2020: A systematic review
Abstract
Background: A year following the onset of the COVID-19 pandemic, new infections and deaths continue to increase in Europe. Serological studies, through providing evidence of past infection, can aid understanding of the population dynamics of SARS-CoV-2 infection.
Objectives: This systematic review of SARS-CoV-2 seroprevalence studies in Europe was undertaken to inform public health strategies including vaccination, that aim to accelerate population immunity.
Methods: We searched the databases Web of Science, MEDLINE, EMBASE, SCOPUS, Cochrane Database of Systematic Reviews and grey literature sources for studies reporting seroprevalence of SARS-CoV-2 antibodies in Europe published between 01/12/2019-30/09/20. We provide a narrative synthesis of included studies. Studies were categorized into subgroups including healthcare workers (HCWs), community, outbreaks, pregnancy and children/school. Due to heterogeneity in other subgroups, we only performed a random effects meta-analysis of the seroprevalence amongst HCWs stratified by their country.
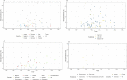
Results: 115 studies were included spanning 17 European countries, that estimated the seroprevalence of SARS-CoV-2 from samples obtained between November 2019 -August 2020. A total of 54/115 studies included HCWs with a reported seroprevalence among HCWs ranging from 0.7% to 45.3%, which did not differ significantly by country. In community studies significant heterogeneity was reported in the seroprevalence between different age groups and the majority of studies reported there was no significant difference by gender.
Conclusion: This review demonstrates a wide heterogeneity in reported seroprevalence of SARS-CoV-2 antibodies between populations. Continued evaluation of seroprevalence is required to understand the impact of public health measures and inform interventions including vaccination programmes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020 [Internet]. [cited 2020 Nov 18]. Available from: https://www.who.int/director-general/speeches/detail/who-director-genera...
-
- Kutti-Sridharan G, Vegunta R, Vegunta R, Mohan BP, Rokkam VRP. SARS-CoV2 in Different Body Fluids, Risks of Transmission, and Preventing COVID-19: A Comprehensive Evidence-Based Review. Int J Prev Med [Internet]. 2020. [cited 2020 Nov 18];11:97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33042494 doi: 10.4103/ijpvm.IJPVM_255_20 - DOI - PMC - PubMed
-
- Anderson EL, Turnham P, Griffin JR, Clarke CC. Consideration of the Aerosol Transmission for COVID-19 and Public Health. Risk Anal [Internet]. 2020. [cited 2020 Nov 18];40(5):902–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32356927 doi: 10.1111/risa.13500 - DOI - PMC - PubMed
-
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet (London, England) [Internet]. 2021. May 1 [cited 2021 Jun 25];397(10285):1603–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33865497 doi: 10.1016/S0140-6736(21)00869-2 - DOI - PMC - PubMed
-
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med [Internet]. 2020. Apr 30 [cited 2020 Nov 18];382(18):1708–20. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2002032 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

